Synthesis method of 2-(2, 6-diethyl-4-methyl benzene) diethyl malonate
A technology of diethyl malonate and synthesis method, which is applied to the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as decomposition of diazonium salts, three wastes in aqueous systems, instability, etc., and achieve Less decomposition, faster reaction speed, and the effect of making up for low temperature kinetic energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
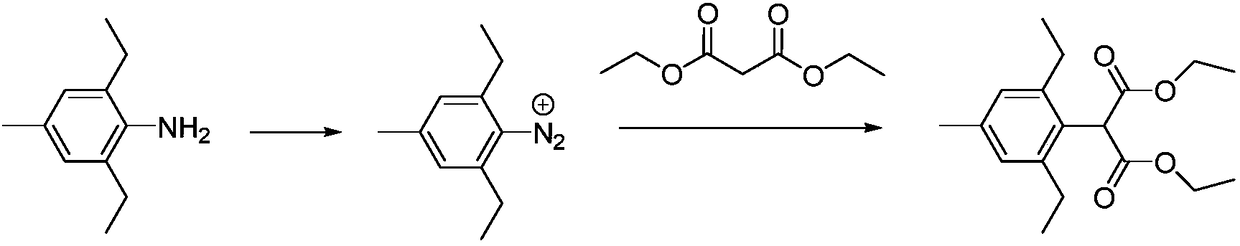
[0030] Step (1), add 500g of acetonitrile into the four-necked flask, put in 163.3g (1mol) 2,6-diethyl-4-methylaniline, 1.6g of cuprous chloride, cool down to 5-10°C, drop 123g (1.05mol) of isoamyl nitrite, the temperature is controlled at 5-10°C during the dropwise addition, after the dropwise addition is completed, the reaction is kept for about 2 hours, and the reaction is completed, and the diazonium solution is kept for later use.
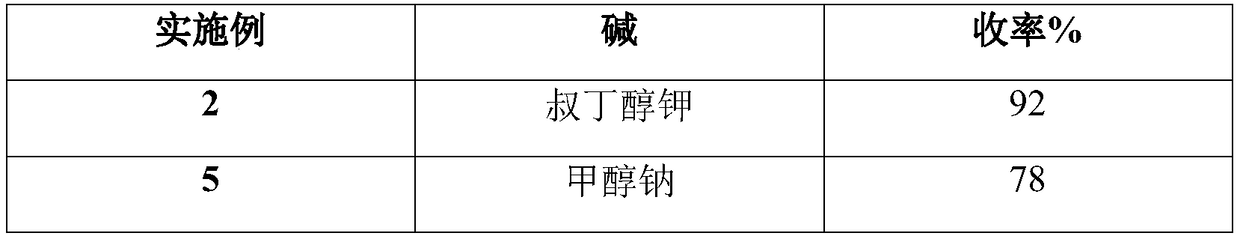
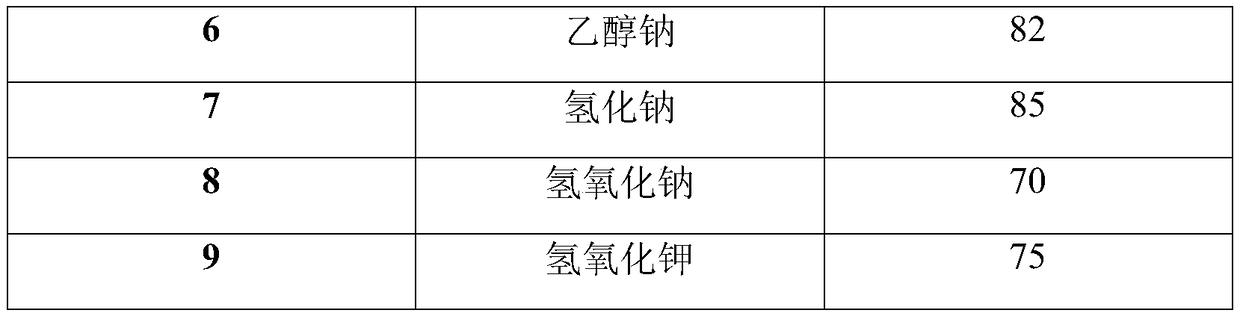
[0031] Step (2), add 176g (1.1mol) of diethyl malonate and 300g of N,N-dimethylformamide into the four-necked flask, cool down to 0-5°C, and add 136g (1.2mol) of t- Potassium butoxide, control the temperature at 0-5°C, after the feeding is completed, slowly raise the temperature to 15-20°C, react for 1 hour, add the diazonium solution prepared in step (1) dropwise to the reaction liquid on the principle of not flushing, control The temperature is 15-20°C. After the dropwise addition is completed, the reaction is kept for 1.5 hours, and the rea...
Embodiment 2
[0033] Step (1), add 500g tetrahydrofuran into the four-neck flask, put in 163.3g 2,6-diethyl-4-methylaniline, 1.6g cuprous iodide, cool down to 5-10°C, add dropwise 123g nitrous acid For isoamyl ester, control the temperature at 5-10°C. After the dropwise addition, keep the reaction for about 2 hours. After the reaction is over, keep the diazonium solution for standby.
[0034] Step (2), add 176g of diethyl malonate and 300g of N,N-dimethylformamide into the four-neck flask, cool down to 0-5°C, add 136g of potassium tert-butoxide in batches, and control the temperature from 0 to 5°C. 5°C, after the feeding is finished, slowly raise the temperature to 15-20°C, react for 1 hour, then dropwise add the diazonium solution prepared in step (1) to the reaction solution, control the temperature at 15-20°C, after the dropwise addition, keep warm for reaction After 1.5 hours, the reaction ended. The reaction solution was added to 1000 g of water, stirred for 0.5 hours, cooled to 0-5° ...
Embodiment 3
[0036] Step (1), add 500g of tetrahydrofuran into the four-neck flask, put in 163.3g of 2,6-diethyl-4-methylaniline, 2.4g of cuprous iodide, cool down to 5-10°C, add dropwise 123g of nitrous acid For isoamyl ester, control the temperature at 5-10°C. After the dropwise addition, keep the reaction for about 2 hours. After the reaction is over, keep the diazonium solution for standby.
[0037] Step (2), add 176g of diethyl malonate and 300g of N,N-dimethylformamide into the four-neck flask, cool down to 0-5°C, add 136g of potassium tert-butoxide in batches, and control the temperature from 0 to 5°C. 5°C, after the feeding is finished, slowly raise the temperature to 15-20°C, react for 1 hour, then dropwise add the diazonium solution prepared in step (1) to the reaction solution, control the temperature at 15-20°C, after the dropwise addition, keep warm for reaction After 1.5 hours, the reaction ended. The reaction solution was added to 1000 g of water, stirred for 0.5 hours, coo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com