Synthesis method of carbazole derivatives and anti-tumor application
A technology of derivatives and indazoles is applied in the field of rapid preparation of indazole derivatives to achieve the effects of strong inhibition effect, simple operation process and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
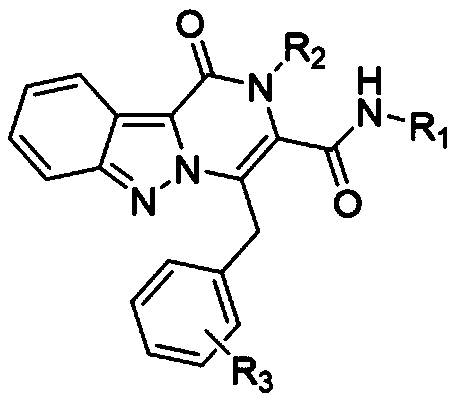
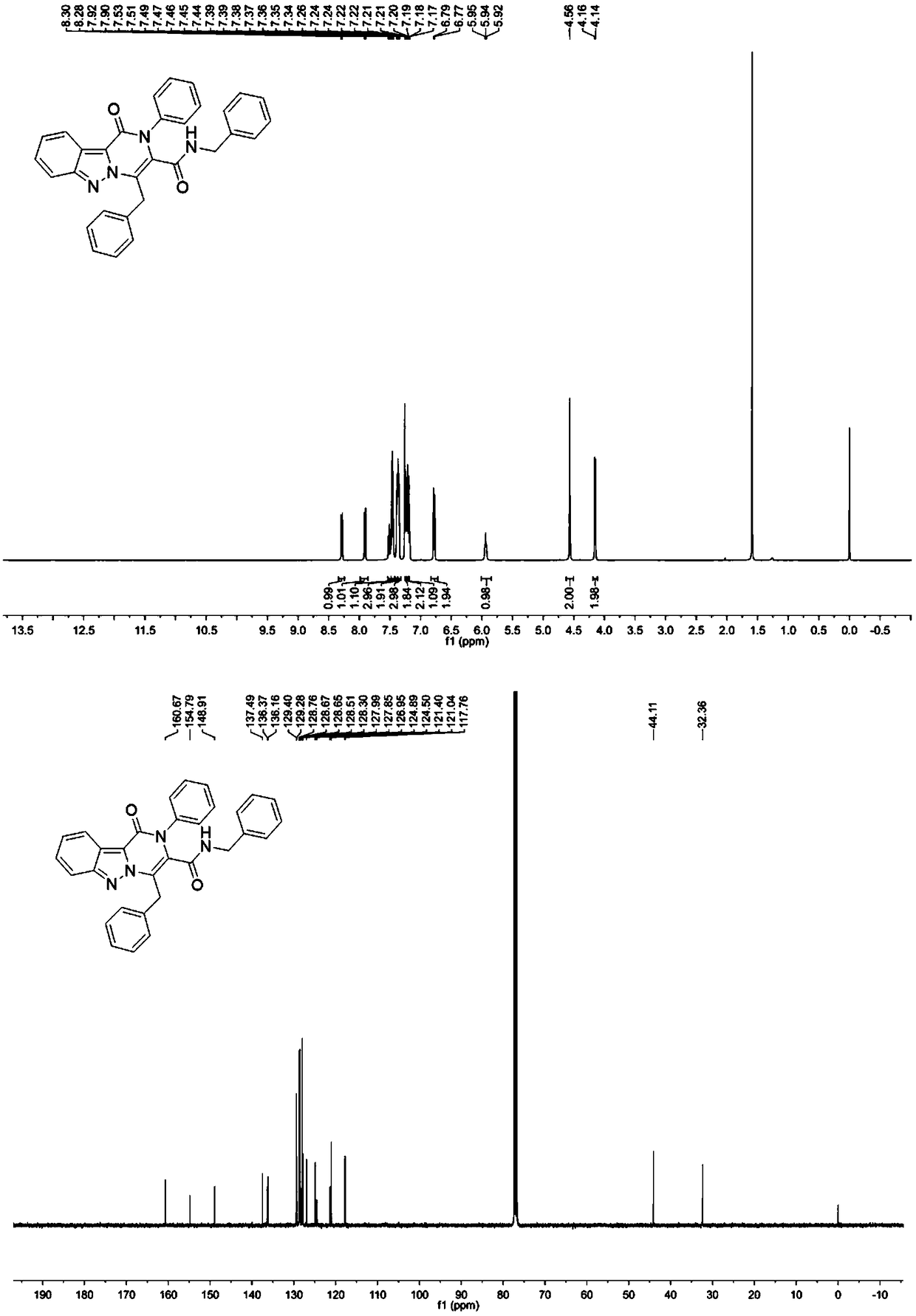
[0043] where R 1 is benzyl, R 2 is aryl, R 3 Synthesis of N, 1,4-dibenzyl-1-oxo-2-phenyl-1,2-dihydropyrazino[1,2-b]indazole-3-carboxamide ,Specific steps are as follows:
[0044] Dissolve 1.0 mmol of 3-phenylpropargylaldehyde in 2 mL of trifluoroethanol in a 5 mL microwave reaction tube, add 1.0 mmol of aniline, stir at room temperature for 10 minutes, and then add 1.0 mmol of 1H-indazole-3-carboxylate acid and 1.0 mmol benzyl isonitrile, stirred overnight at room temperature. TLC detection reaction. After the reaction was complete, the solvent was removed, the residue was dissolved in DMF (5.0 mL), and placed in a microwave reactor for 110 oC for 20 minutes. After the reaction was completed, it was cooled to room temperature, the reaction solution was poured into 15 mL ethyl acetate, washed with saturated brine, the organic phase was dried over anhydrous magnesium sulfate, concentrated, and separated by gradient elution with ethyl acetate / n-hexane (0-60%) to obtain The...
Embodiment 2
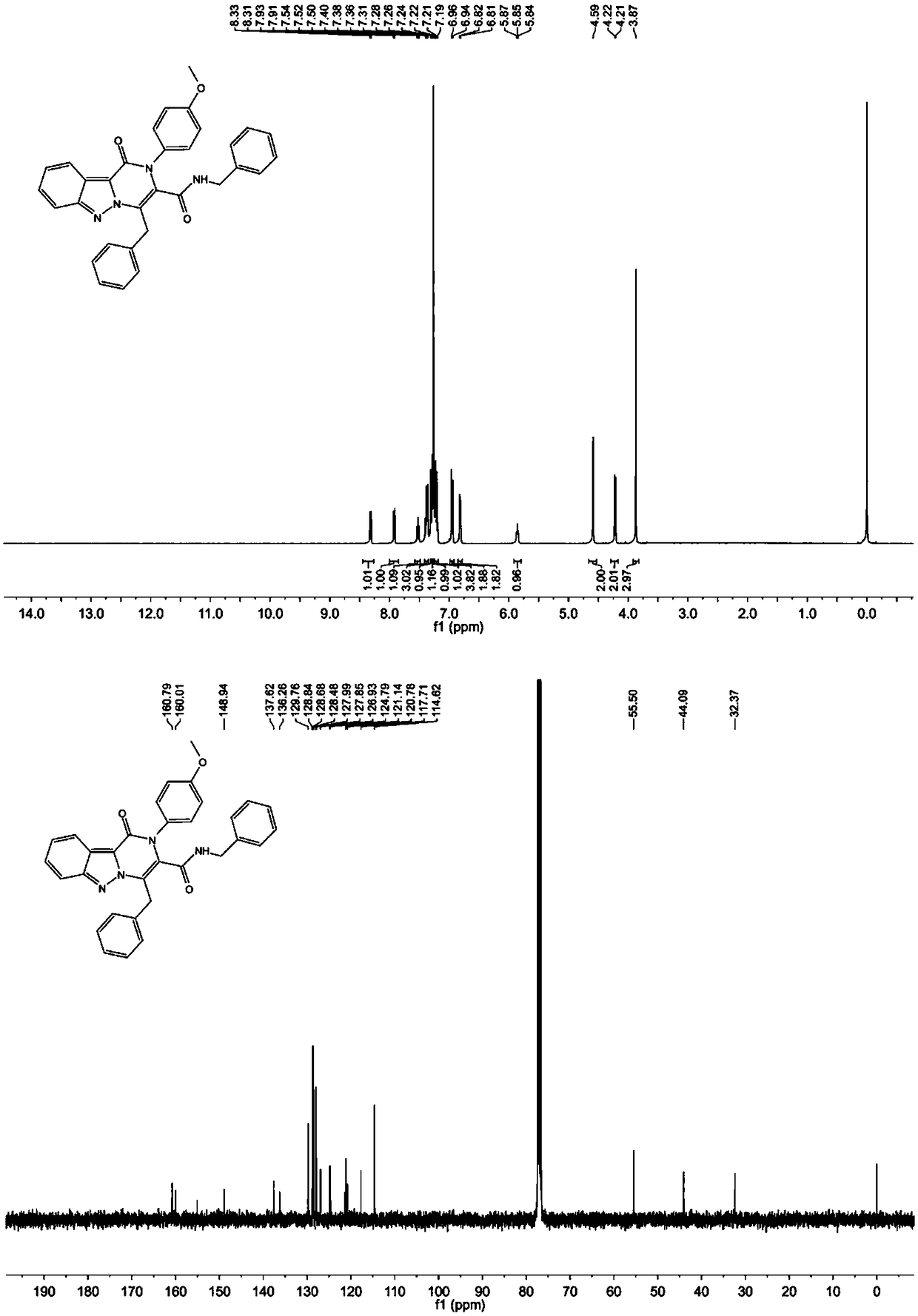
[0047] where R 1 is benzyl, R 2 is aryl, R 3 is a hydrogen atom, namely N,1,4-dibenzyl-2-(4-methoxyphenyl)-1-oxo-1,2-dihydropyrazino[1,2-b]indazole The synthesis of -3-carboxamide, concrete steps are as follows:
[0048] In a 5 mL microwave reaction tube, dissolve 1.0 mmol of 3-phenylpropargylaldehyde in 2 mL of trifluoroethanol, add 1.0 mmol of p-methoxyaniline, stir at room temperature for 10 minutes, and then add 1.0 mmol of 1H-indole Azole-3-carboxylic acid and 1.0 mmol benzyl isonitrile were stirred overnight at room temperature. TLC detection reaction. After the reaction was complete, the solvent was removed, the residue was dissolved in DMF (5.0 mL), and placed in a microwave reactor for 110 o C for 20 minutes. After the reaction was completed, it was cooled to room temperature, the reaction solution was poured into 15 mL ethyl acetate, washed with saturated brine, the organic phase was dried over anhydrous magnesium sulfate, concentrated, and separated by gradien...
Embodiment 3
[0051] where R 1 is phenethyl, R 2 is aryl, R 3 is a hydrogen atom, namely 4-benzyl-1-oxo-N-phenethyl-2-phenyl-1,2-dihydropyrazino[1,2-b]indazole-3-carboxamide Synthesis, the specific steps are as follows:
[0052] In a 5 mL microwave reaction tube, dissolve 1.0 mmol of 3-phenylpropargylaldehyde in 2 mL of trifluoroethanol, add 1.0 mmol of aniline, stir at room temperature for 10 minutes, and then add 1.0 mmol of 1H-indazole-3- Carboxylic acid and 1.0 mmol phenethylisonitrile were stirred overnight at room temperature. TLC detection reaction. After the reaction was complete, the solvent was removed, the residue was dissolved in DMF (5.0 mL), and placed in a microwave reactor for 110 o C for 20 minutes. After the reaction was completed, it was cooled to room temperature, the reaction solution was poured into 15 mL ethyl acetate, washed with saturated brine, the organic phase was dried over anhydrous magnesium sulfate, concentrated, and separated by gradient elution with e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com