Tetrapeptide and application thereof
An amino acid and sequence technology, applied in the direction of peptides, specific peptides, immunoglobulins, etc., can solve problems such as ligand shedding pollution, antibody separation and purification technology needs to be improved, and Protein A/G is easy to inactivate, and achieves the effect of simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0026] Example 3 Antibody Adsorption Peptide Separation and Purification of Antibody
[0027] 1) Prepare 20mmol / L Hepes buffer solution, pH7.4, NaCl content 0.15M. Set the flow rate to 1mL / min.
[0028] 2) Use the liquid prepared in 1) as the mobile phase, and run the AKTA protein purification system.
[0029] 3) Take 2 mL of the affinity medium in Example 2 to fill the separation column.
[0030] 4) Load 2 mL of antibody IgG fermentation broth.
[0031] 5) Prepare 0.1M HCl-Gly buffer at pH 2 as the eluent.
[0032] 6) Load 6 mL of the eluate respectively, and collect the eluate.
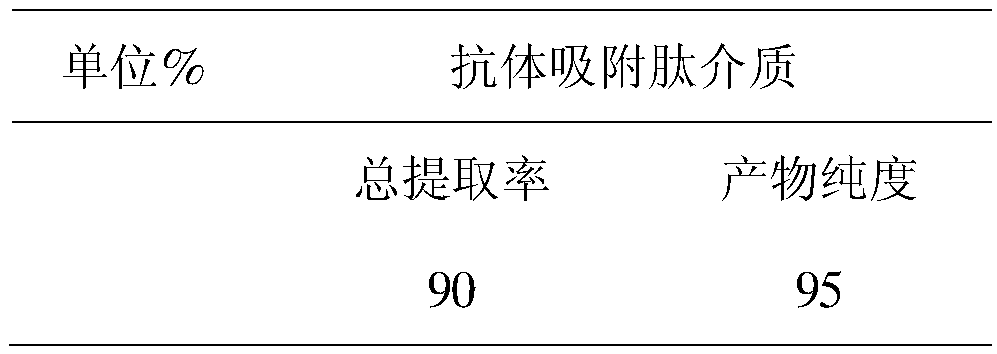
[0033] 7) Measure the antibody content and purity in the antibody fermentation broth and the eluate of the novel separation short peptide medium, and calculate the extraction rate.
[0034] Table 1 Adsorption characteristics of the new medium for separating short peptides
[0035]
[0036] It can be seen from Table 1 that the polypeptides of the present invention can specifically bind to an...
Embodiment 4
[0037] Embodiment 4 adsorption medium preparation
[0038] 1) Dissolve 10 mg of the polypeptide prepared in Example 1 in 5 mL of Hepes buffer, pH 7.4.
[0039] 2) Take 2 mL of Sepharose gel activated by sulfhydryl group of GE Company.
[0040] 3) The above two were mixed and reacted for 8 hours.
[0041] 4) After the reaction is completed, the gel particles are washed with Hepes buffer to obtain an affinity medium.
Embodiment 5
[0042] Example 5 Antibody Adsorption Peptide Specific Adsorption Antibody
[0043] 1) Prepare 20mmol / L Hepes buffer solution, pH7.4, NaCl content 0.15M. Set the flow rate to 1mL / min.
[0044] 2) Use the liquid prepared in 1) as the mobile phase, and run the AKTA protein purification system.
[0045] 3) Take 2mL of the affinity medium and 2mL of the Protein A medium in Example 4 to fill the separation column respectively.
[0046] 4) Load 2 mL of serum respectively.
[0047] 5) Prepare 0.1M HCl-Gly buffer at pH 2 as the eluent.
[0048] 6) Load 6 mL of the eluent, respectively, and collect the effluent and eluate.
[0049] 7) Measure the antibody IgG content and purity in the serum, effluent and eluate respectively, and calculate the extraction rate.
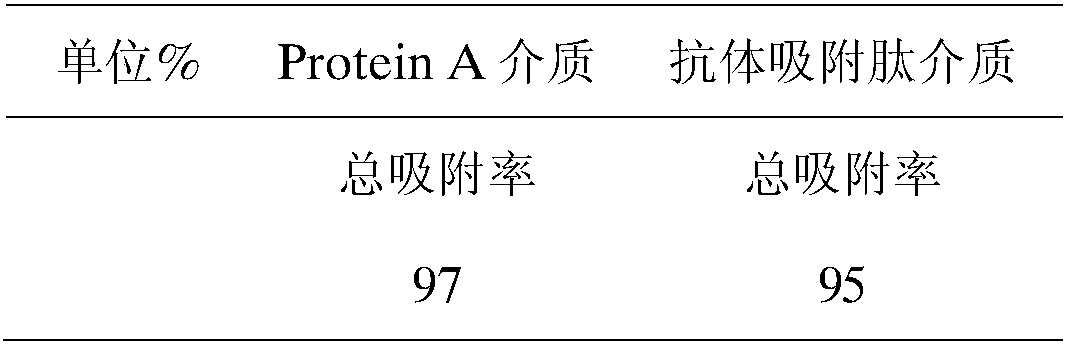
[0050] Table 2 Adsorption characteristics of the new medium for separating short peptides
[0051]
[0052] It can be known from Table 2 that the polypeptides of the present invention can specifically bind to antibodies....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com