Method for preparing 1,6-adipaldehyde through selective oxidation of cyclohexene
A technology of adipaldehyde and cyclohexene, applied in the field of selective oxidation of cyclohexene to prepare 1,6-adipaldehyde, which can solve the problems of poor selectivity and achieve the effects of environmental friendliness, high reactivity and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of isosterveol derivative amino alcohols: Isosterveol derivative amino alcohols were synthesized according to the literature (Lai Hongtao, Synthesis of novel diamine chiral ligands and their application in asymmetric organic catalysis Research, 2016, Master Thesis of Zhengzhou University).
[0023] Synthesis of isosterveol derivative aminoalcohol glyoxal condensate: Add 20mL methanol and 7.5g (0.02mol) isosterveol derivative aminoalcohol into a 100mL three-neck flask, stir and drop glyoxal 1.5 g (0.01 mol), stirred at room temperature for 8 hours, the precipitate was suction-filtered, washed with methanol, and dried in vacuum at 50°C to obtain 5.8 g of isosteviol derivative amino alcohol glyoxal condensate, yield 75.1%. Its structural formula is:
[0024] ;
[0025] 1H NMR (400 MHz, CDCl 3 , TMS): δ7.5 (d, 2H), 4.06–4.11 (m, 4H), 3.82 (d,2H),, 3.33 (m, 4H), 3.04 (d, 2H), 2.49 (s, 2H) , 2.15 (d, 2H), 1.42–1.83(m,18H), 1.83–1.09 (m, 14H) , 1.27 (s, 6H), 1....
Embodiment 2
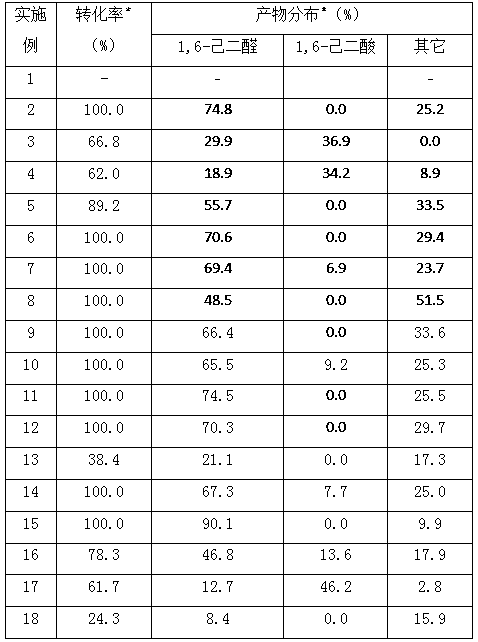
[0031] Add 8.2 g (0.1 mol) cyclohexene, 0.95 g (0.001 mol) cobalt complex of isosteviol derivatives, 20 mL tetrahydrofuran, 12.9 g (0.1 mol) tert-butanol peroxide into the autoclave, and feed oxygen Until the pressure of the reactor is 0.4 MPa, the reaction temperature is 160° C., and the reaction time is 180 minutes. The specific reaction results are shown in Table 1.
[0032] 1,6-Hexanal (C 6 h 10 o 2 ): 1H NMR (400 MHz, CDCl 3 , TMS): δ9.7 (t,2H), 2.4 (m,4H), 1.6 (m, 4H); GC-MS: [M] + 114, [M-29] + 85.
Embodiment 3
[0034] Add 8.2 g (0.1 mol) of cyclohexene, 0.94 g (0.001 mol) of manganese complex of isosteviol derivatives, 20 mL of tetrahydrofuran, 12.9 g (0.1 mol) of tert-butanol peroxide into the autoclave, and feed oxygen The pressure of the reactor was 0.4MPa, the reaction temperature was 160°C, and the reaction time was 180 minutes. The specific reaction results are shown in Table 1.
[0035] 1,6-Hexanal (C 6 h 10 o 2 ): 1H NMR (400 MHz, CDCl 3 , TMS): δ9.7 (t,2H), 2.4 (m,4H), 1.6 (m, 4H); GC-MS: [M] + 114, [M-29] + 85.
[0036] 1,6-Hexanedioic acid (C 6 h 10 o 4 ): Mp 151.2~152.3℃; 1H NMR (400 MHz, CDCl3, TMS): δ2.2 (t, 4H), 1.6 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com