A kind of preparation method of ethyl trifluoroacetate
A technology of ethyl trifluoroacetate and trifluoroacetic acid, applied in the field of preparation of ethyl trifluoroacetate, can solve the problems of high equipment requirements, adverse environmental impact, low yield, etc., and achieves reduced corrosiveness, reduced dosage, and reduced reaction high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
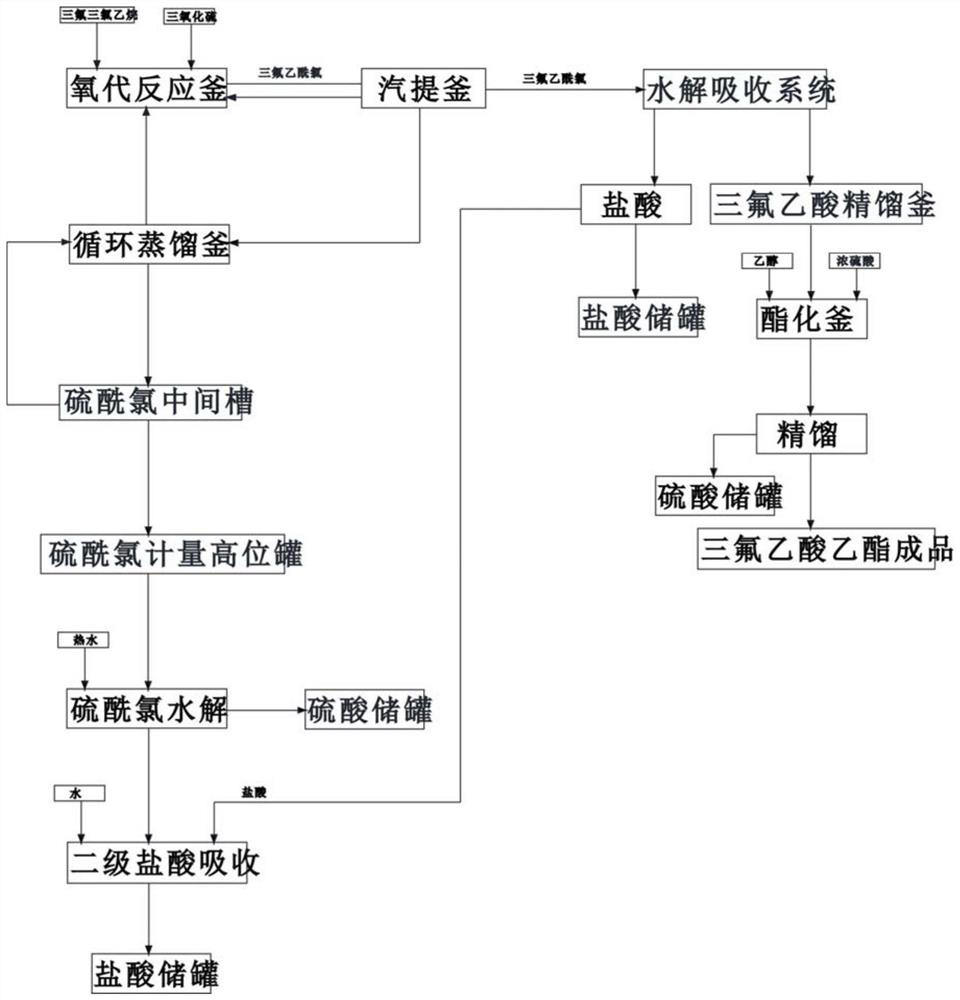
[0026] 1) Pass 2kg of sulfur trioxide from the storage tank through the oxygen reaction kettle through the internal pressure, and pass 2kg of trifluorotrichloroethane through the oxygen reaction kettle, wherein the sulfur trioxide is passed through a jacket at about 70°C Heating with hot water, controlling the temperature in the oxo reactor to 58°C, and reacting for 20 minutes;
[0027] 2) Pass 3kg of sulfur trioxide from the storage tank through the oxo reactor through internal pressure, pass 6kg of trifluorotrichloroethane through the oxo reactor, control the temperature in the oxo reactor to be 60°C, and react 10 minutes;
[0028] 3) The reaction product trifluoroacetyl chloride in step 1) and 2) and the by-product sulfuryl chloride, pyrothionyl chloride, along with part of the raw materials sulfur trioxide, trifluorotrichloroethane, enter the vapor phase from the gas phase outlet of the oxo reactor. Stripping still, carry out stripping condensation separation at 63 ℃;
...
Embodiment 2
[0041] 1) Pass 4kg of sulfur trioxide from the storage tank through the oxygen reaction kettle through the internal pressure, and pass 4kg of trifluorotrichloroethane through the oxygen reaction kettle, wherein the sulfur trioxide is passed through a jacket at about 70°C Heating with hot water, controlling the temperature in the oxo reactor to 60°C, and reacting for 25 minutes;
[0042] 2) Pass 2kg of sulfur trioxide from the storage tank through the oxo reactor through the internal pressure, pass 4kg of trifluorotrichloroethane through the oxo reactor, control the temperature in the oxo reactor to be 62°C, and react 15 minutes;
[0043] 3) The reaction product trifluoroacetyl chloride in step 1) and 2) and the by-product sulfuryl chloride, pyrothionyl chloride, along with part of the raw materials sulfur trioxide, trifluorotrichloroethane, enter the vapor phase from the gas phase outlet of the oxo reactor. Stripping still, carry out stripping condensation separation at 64 ℃;...
Embodiment 3
[0056] 1) Pass 2.5kg of sulfur trioxide from the storage tank through the oxo reaction kettle through internal pressure, and pass 2.5kg of trifluorotrichloroethane through the oxo reaction kettle, wherein the sulfur trioxide is passed through 70°C with a jacket Heating with hot water from left to right, controlling the temperature in the oxo reactor to 61°C, and reacting for 28 minutes;
[0057] 2) Pass 2kg of sulfur trioxide from the storage tank through the oxo reactor through internal pressure, pass 4kg of trifluorotrichloroethane through the oxo reactor, control the temperature in the oxo reactor to be 64°C, and react 25 minutes;
[0058] 3) The reaction product trifluoroacetyl chloride in step 1) and 2) and the by-product sulfuryl chloride, pyrothionyl chloride, along with part of the raw materials sulfur trioxide, trifluorotrichloroethane, enter the vapor phase from the gas phase outlet of the oxo reactor. Stripping still, carry out stripping condensation separation at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com