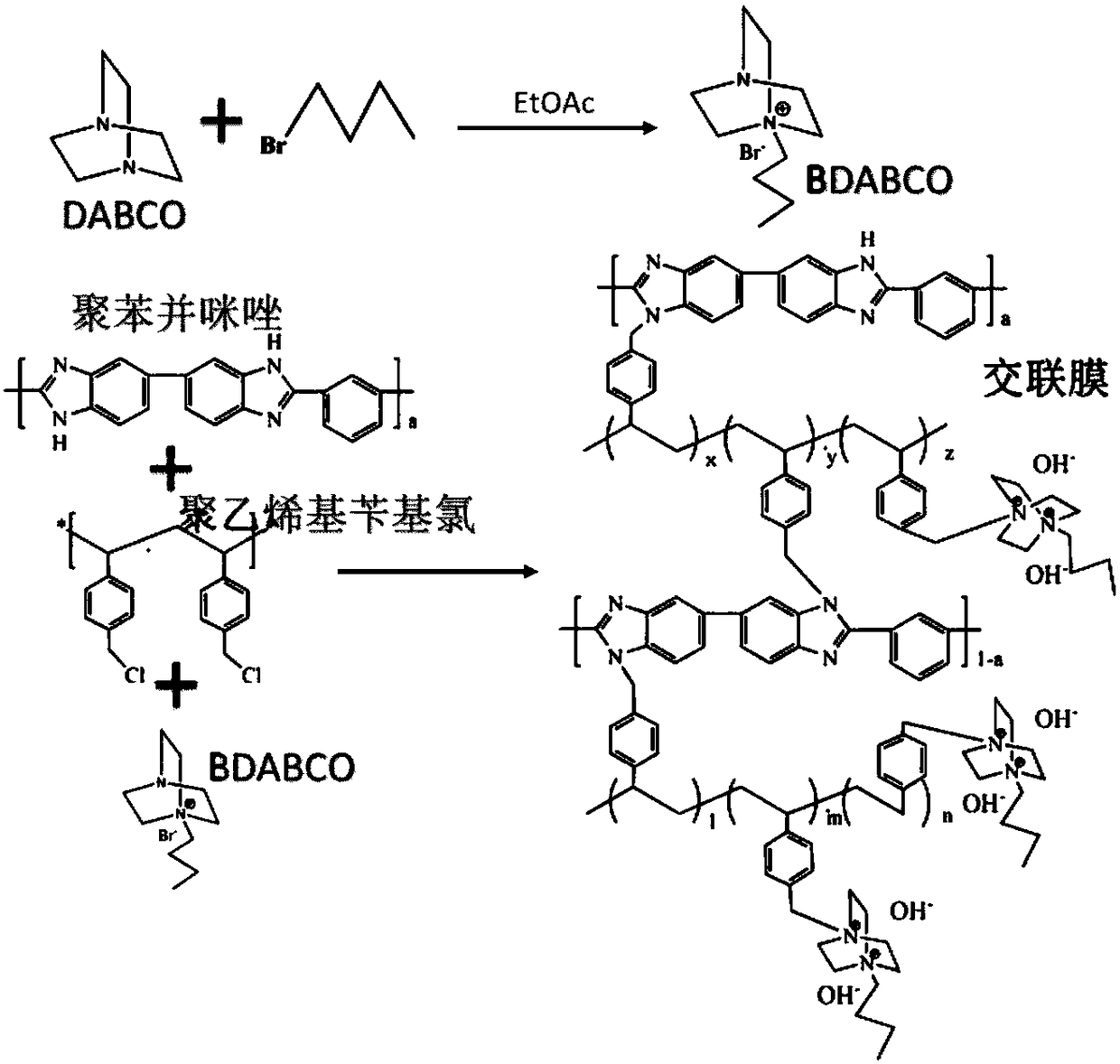
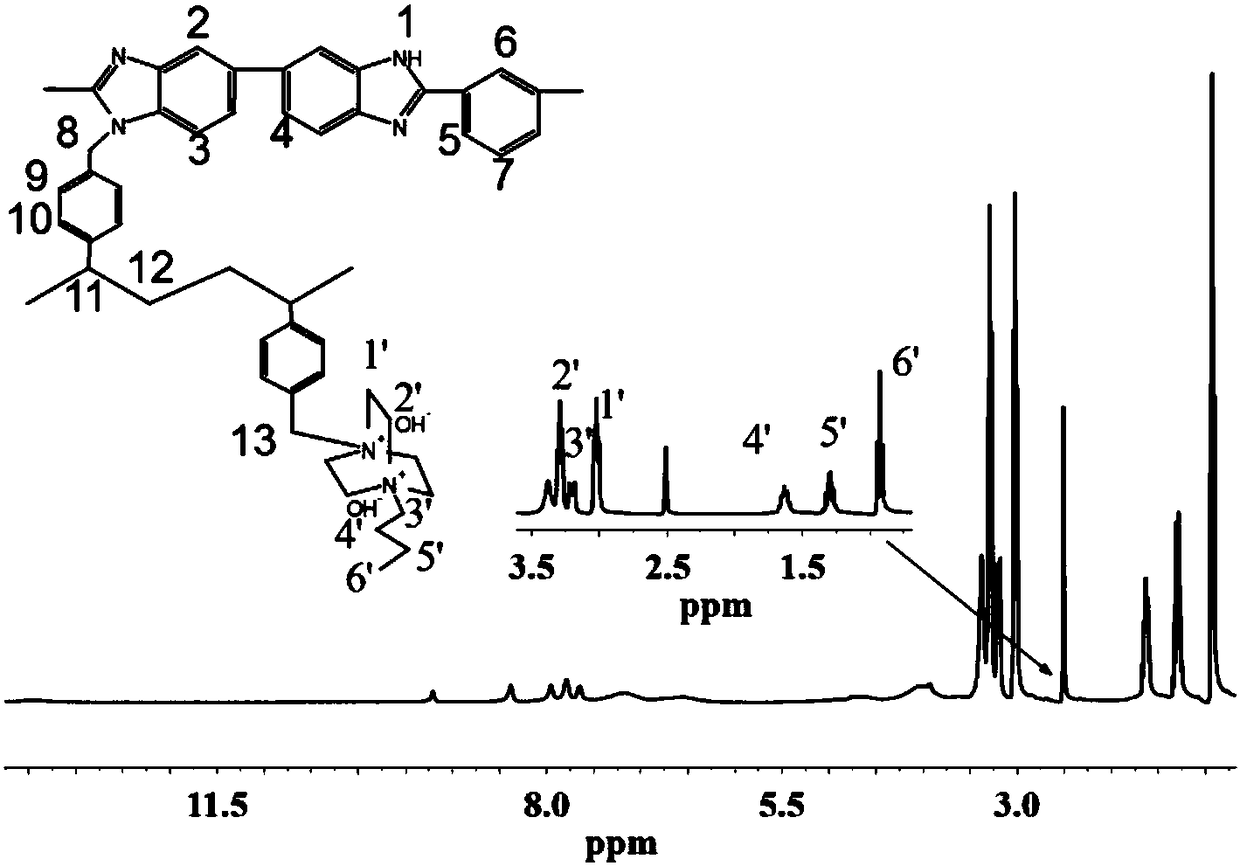
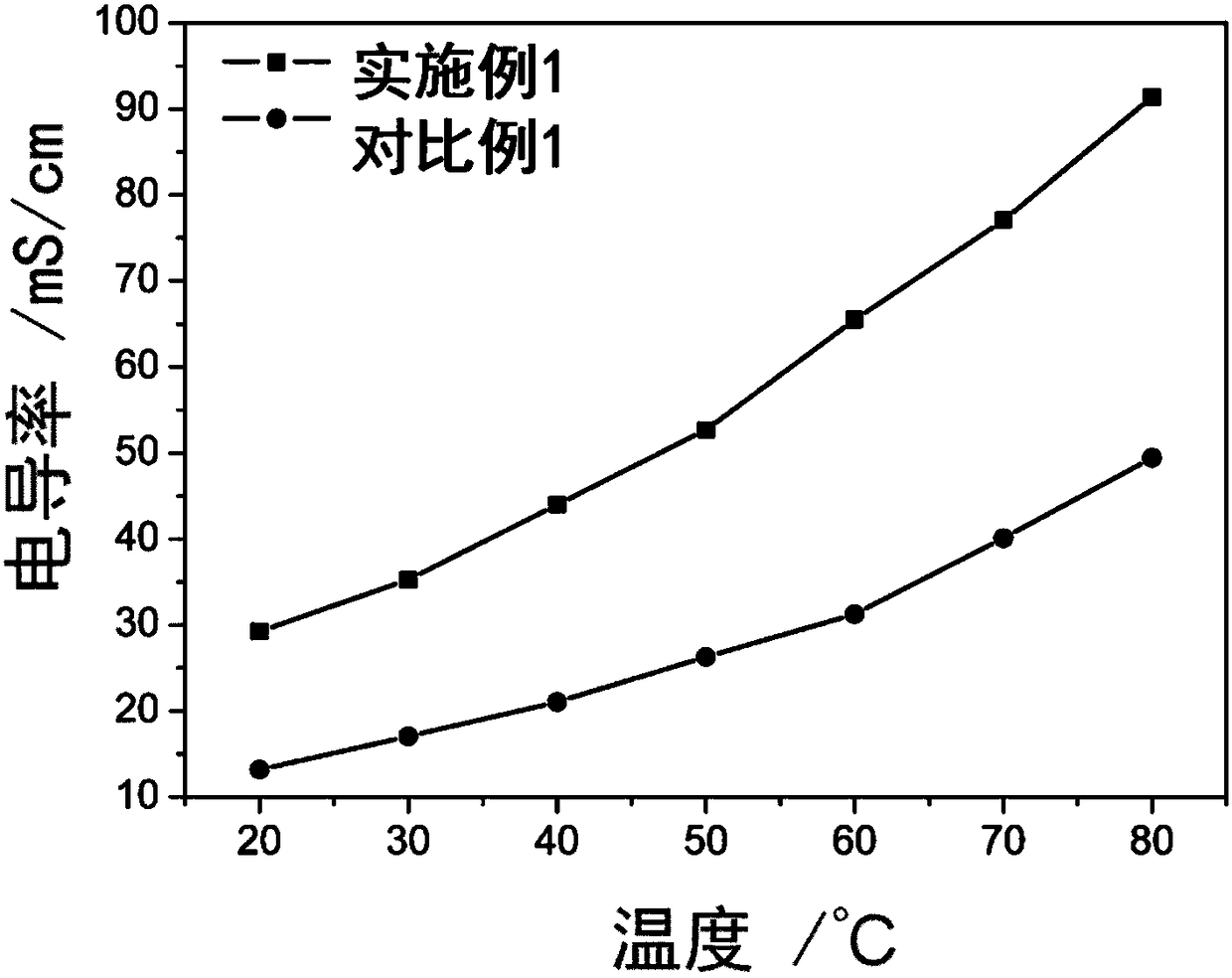
Cross-linked polybenzimidazole basic anion exchange membrane, and preparation and application thereof
A polybenzimidazole, alkaline anion technology, applied in the field of alkaline anion exchange membrane fuel cells, can solve the problems of poor membrane dimensional stability, low conductivity of alkaline anion exchange membrane, etc., achieve environmental friendliness, improve mechanical strength and Dimensional stability, the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthesis process of 1-butyl-1,4-diazabicyclo[2.2.2]octane is as follows:
[0038] 1) Add 10g of 1,4-diazabicyclo[2.2.2]octane into 100mL ethyl acetate, stir and dissolve at room temperature, then add n-bromobutane, the number of moles added is 1,4-diazepine Heterobicyclo[2.2.2]octane 1.2 times, stirred overnight at room temperature;
[0039] 2) Filter the white suspension, wash it several times with ethyl acetate, and immediately dry it in a vacuum oven at 60°C to obtain a white powder of 1-butyl-1,4-diazabicyclo[2.2.2] octane.
[0040] The preparation method of 1-butyl-1,4-diazabicyclo[2.2.2]octane functionalized cross-linked polybenzimidazole basic anion exchange membrane comprises the following steps,
[0041] 1) Prepare a polybenzimidazole NMP solvent solution with a concentration of 1 wt%, and add polyvinylbenzyl chloride at room temperature, wherein the molar ratio of polybenzimidazole: polyvinylbenzyl chloride is 3:2 , stirred at room temperature for more...
Embodiment 2
[0057] The synthesis process of 1-butyl-1,4-diazabicyclo[2.2.2]octane is as follows:
[0058] 1) Add 15g of 1,4-diazabicyclo[2.2.2]octane into 150mL ethyl acetate, stir and dissolve at room temperature, then add n-bromobutane, the number of moles added is 1,4-diazepine Heterobicyclo[2.2.2]octane 1.3 times, stirred overnight at room temperature;
[0059] 2) Filter the white suspension, wash it several times with ethyl acetate, and immediately dry it in a vacuum oven at 60°C to obtain a white powder of 1-butyl-1,4-diazabicyclo[2.2.2] octane.
[0060] The preparation method of 1-butyl-1,4-diazabicyclo[2.2.2]octane functionalized cross-linked polybenzimidazole basic anion exchange membrane comprises the following steps,
[0061] 1) Prepare a NMP solvent solution of polybenzimidazole with a concentration of 2wt%, and add polyvinylbenzyl chloride at room temperature, wherein the molar ratio of polybenzimidazole: polyvinylbenzyl chloride is 1:1 , stirred at room temperature for mo...
Embodiment 3
[0068] The synthesis process of 1-hexyl-1,4-diazabicyclo[2.2.2]octane is as follows:
[0069] 1) Add 20g of 1,4-diazabicyclo[2.2.2]octane into 300mL of ethyl acetate, stir and dissolve at room temperature, then add n-bromohexane, the number of moles added is 1,4-diaza 1.5 times that of bicyclo[2.2.2]octane, stirred overnight at room temperature;
[0070] 2) Filter the white suspension, wash it several times with ethyl acetate, and immediately dry it in a vacuum oven at 60°C to obtain a white powder of 1-hexyl-1,4-diazabicyclo[2.2.2]octane alkyl.
[0071] 1-hexyl-1,4-diazabicyclo[2.2.2]octane functionalized cross-linked polybenzimidazole basic anion exchange membrane preparation method, comprising the following steps,
[0072] 1) Configure a DMAc solvent solution of polybenzimidazole with a concentration of 2 wt%, and add polyvinylbenzyl chloride at room temperature, wherein the molar ratio of polybenzimidazole: polyvinylbenzyl chloride is 3:2 , stirred at room temperature f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com