Preparation method and application of polyurethane derivative containing triarylamine structure and tetraphenylethylene group
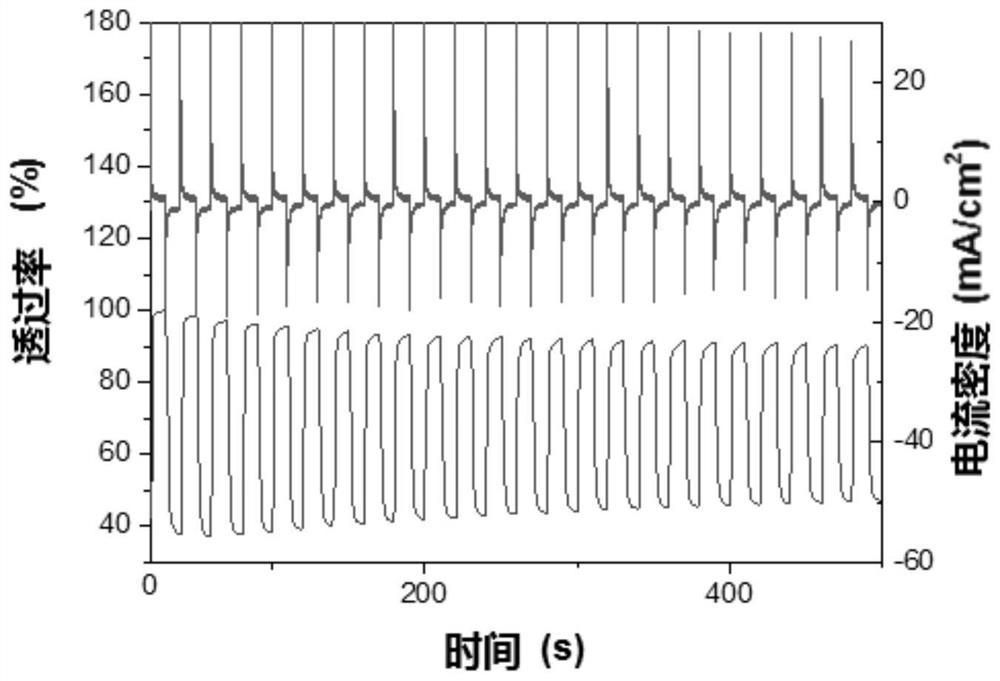
A technology of polyurethane derivatives and tetraphenylene, which is applied in chemical instruments and methods, fluorescence/phosphorescence, instruments, etc., can solve the problems of few functional types of multifunctional materials, achieve good wetting ability, high temperature resistance, and increase dissolution. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
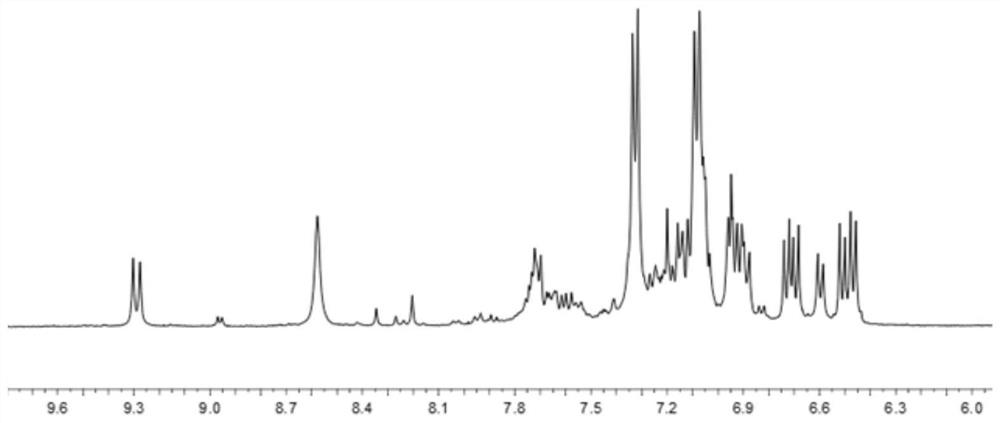
[0044] Specific implementation mode one: the present embodiment contains the polyurethane derivative of triarylamine structure and tetraphenylethylene group, it is characterized in that the polyurethane derivative containing triarylamine structure and tetraphenylethylene group is containing N,N'-bis(4 -Aminophenyl)-N,N'-di-2-naphthyl-1,4-phenylenediamine and tetraphenylethylene group polyurethane derivatives or containing N,N'-bis(4-aminophenyl) - Polyurethane derivatives of N,N'-di-2-naphthyl-1,4-biphenylenediamine groups and tetraphenylethylene groups;
[0045] The structural formula of the polyurethane derivative containing N,N'-bis(4-aminophenyl)-N,N'-di-2-naphthyl-1,4-phenylenediamine and tetraphenylethylene group is as follows:
[0046] In the formula, n is an integer of 3 to 10;
[0047] The structural formula of polyurethane derivatives containing N,N'-bis(4-aminophenyl)-N,N'-di-2-naphthyl-1,4-biphenylenediamine groups and tetraphenylethylene groups is as follows :...
specific Embodiment approach 2
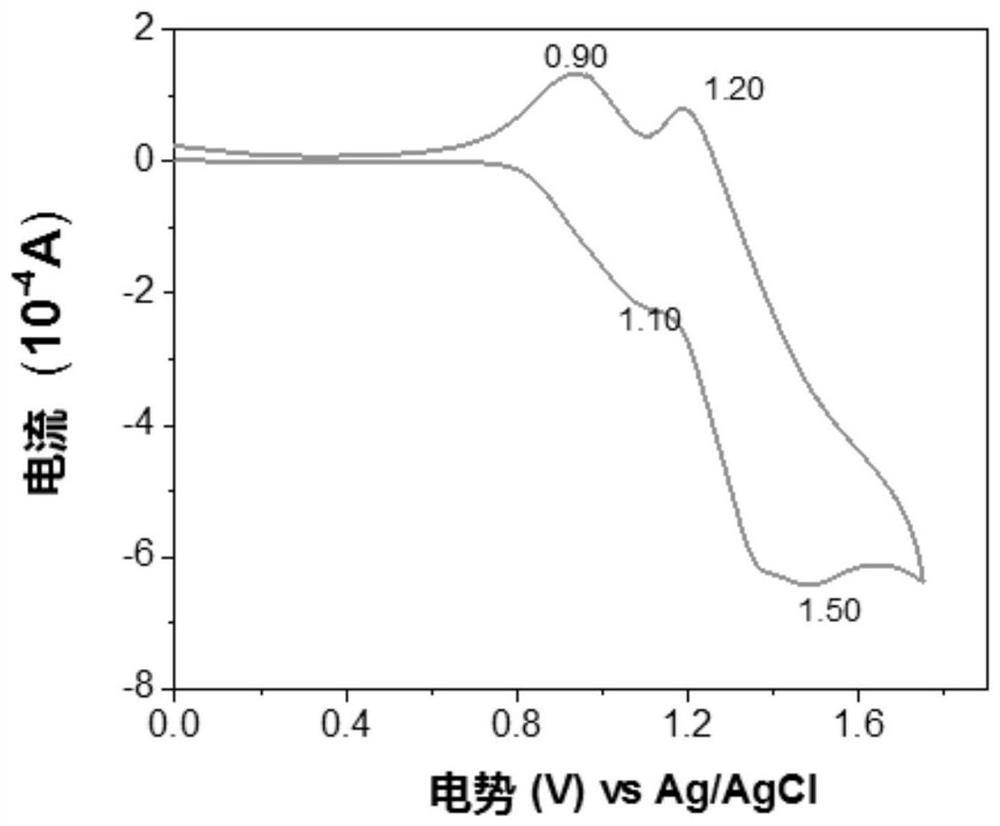
[0053] Specific implementation mode two: the method for the polyurethane derivative containing triarylamine structure and tetraphenylethylene group in this implementation mode is as follows: 1. 1,2-bis(4-hydroxyphenyl)-1,2-diphenylethylene , 4,4′-diphenylmethane diisocyanate and the solvent N,N-dimethylacetamide (DMAC) were mixed, stirred and refluxed for 5-15 hours at room temperature, and the stirring speed was 800r / min~900r / min to obtain the precursor ; Two, will contain N, N'-bis(4-aminophenyl)-N,N'-two-2-naphthyl-1,4-phenylenediamine or N,N'-bis(4-aminophenyl) base)-N,N'-di-2-naphthyl-1,4-biphenyldiamine diamine monomer is added to the precursor prepared in step 1, the temperature is raised to 70-120°C, stirred and refluxed for 5-15h, Pour into methanol after cooling, then filter, and vacuum-dry the obtained solid phase to obtain a polyurethane derivative containing a triarylamine structure and a tetraphenylethylene group;
[0054] Which contains N,N'-bis(4-aminophenyl)-...
specific Embodiment approach 3
[0056] Specific embodiment 3: The difference between this embodiment and specific embodiment 2 is that it contains N,N'-bis(4-aminophenyl)-N,N'-di-2-naphthyl-1,4-benzenedi Amine or N,N'-bis(4-aminophenyl)-N,N'-di-2-naphthyl-1,4-biphenylenediamine diamine monomer, 1,2-bis(4- The molar ratio of hydroxyphenyl)-1,2-diphenylethylene to 4,4'-diphenylmethane diisocyanate is 2:1:2. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com