Excision of retroviral nucleic acid sequences
A retrovirus and nucleic acid sequence technology, applied in the field of excision of retrovirus nucleic acid sequences, can solve problems such as inability to eliminate viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0203] The methods of the invention can be expressed in the manufacture of medicaments. Accordingly, the present invention includes the use of the agents and compositions described herein for the manufacture of a medicament. The compounds described herein are useful in therapeutic compositions and regimens or in the manufacture of medicaments for the treatment of the diseases or conditions described herein.
[0204] Any of the compositions described herein can be administered to any part of the host's body for subsequent delivery to target cells. Compositions can be delivered to the brain, cerebrospinal fluid, joints, nasal mucosa, blood, lungs, intestines, muscle tissue, skin, or peritoneal cavity of a mammal, but are not limited thereto. In terms of route of delivery, the compositions can be injected intravenously, intracranially, intraperitoneally, intramuscularly, subcutaneously, intramuscularly, rectally, intravaginally, intrathecally, intratracheally, intradermally or t...
Embodiment 1
[0231] Example 1: Excision of HIV-1 DNA by Gene Editing: In Vivo Studies
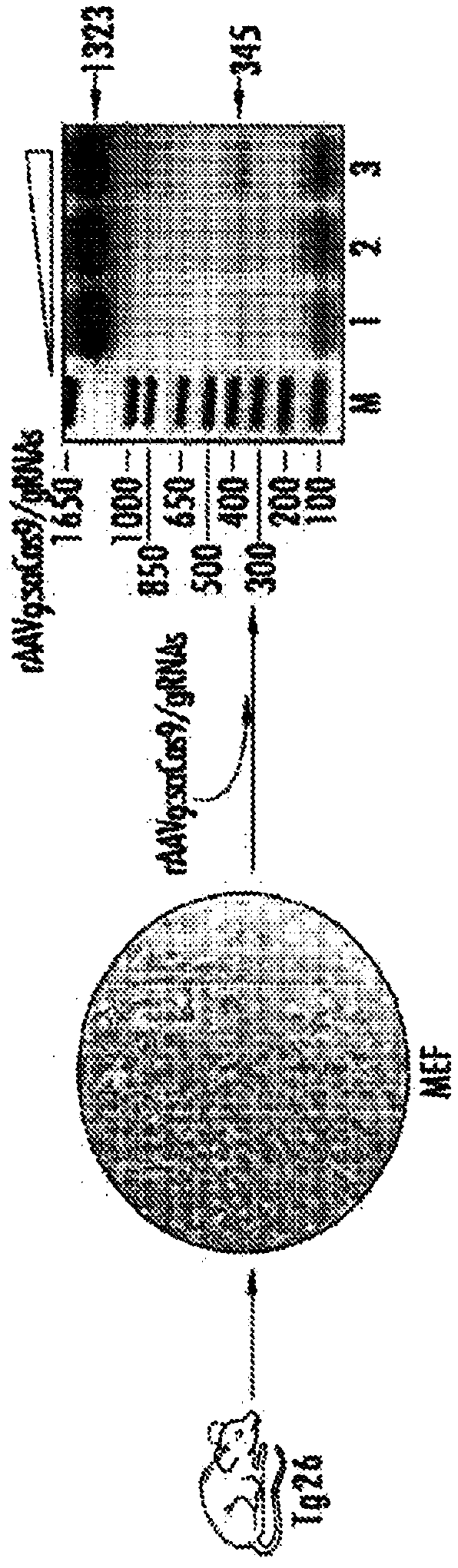
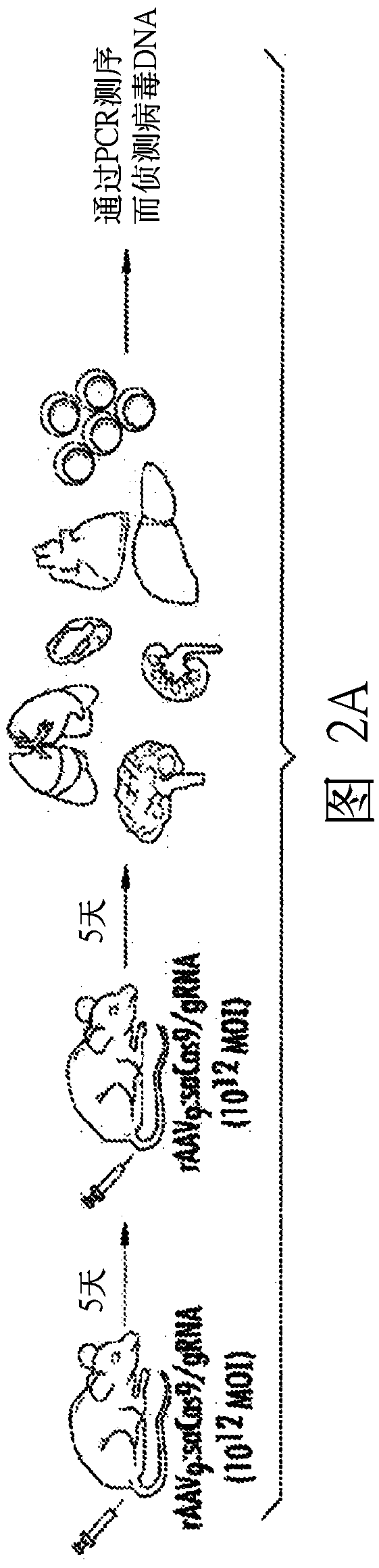
[0232] A CRISPR / Cas9 gene editing strategy was developed to eliminate integrated HIV-1 DNA sequences from latently infected human cells and animal disease models. The efficiency of HIV-1 DNA elimination was studied from mouse tissues carrying the viral genome.
[0233] Materials and methods
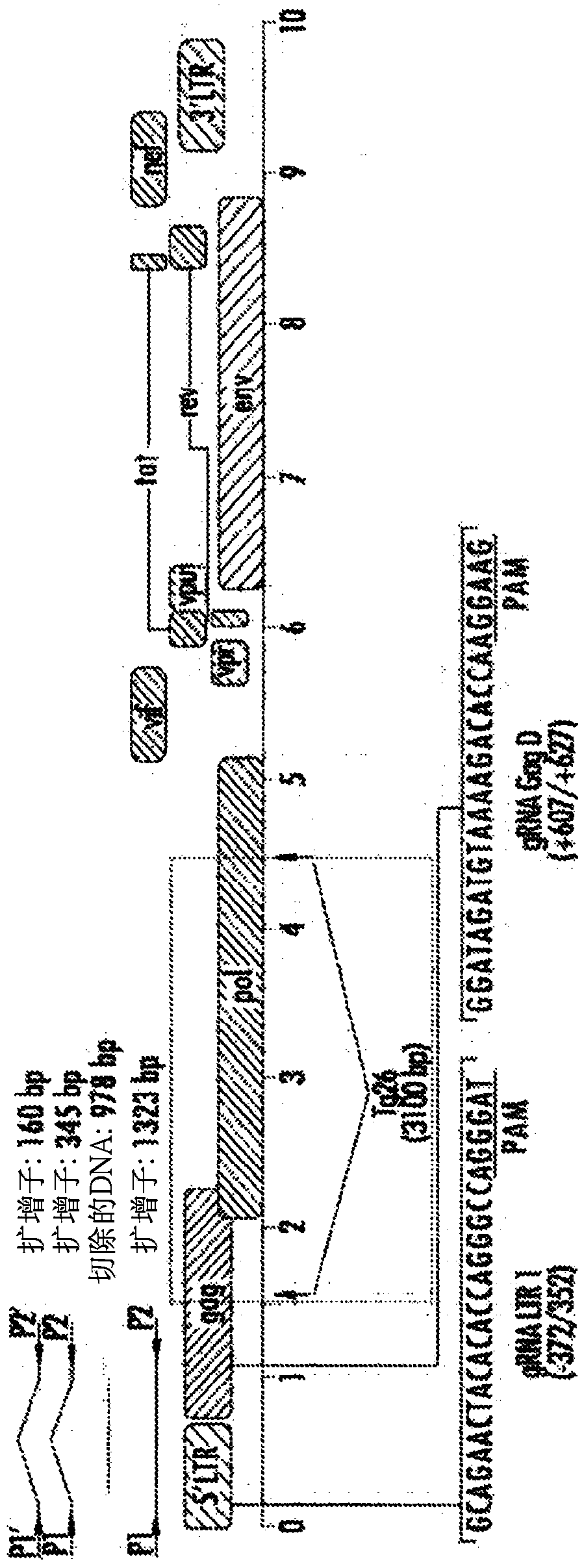
[0234] Construction of AAV9 delivery vector. The background plastid used to generate the AAV delivery system was px601-AAV-CMV::NLS-SaCas9-NLS-3xHA-bGHpA;U6::Bsa1-sgRNA, abbreviated as "pX601" (Ran FA et al., Nature 2015; 520 ):186-191). Oligonucleotides containing the DNA sequences corresponding to the LTR and Gag targets were cloned in front of the U6 promoter at the Bsa1 site in pX601. The gRNA sequences targeting LTR and Gag are shown in Figure 1A In, the PAM sequences are in bold as shown below: gRNA LTR-1: GGA TCA GAT ATC CAC TGA CCT TTG GAT; gRNA Gag D: GGA TAG ATG TAA AAGACA CCA AGG AGG. After confirm...
Embodiment 2
[0248] Example 2: In vivo eradication of HIV provirus by SaCas9 and polyploid sgRNA in a preclinical animal model.
[0249] In this study, combinations of sgRNAs targeting conserved HIV-1 regulatory and structural regions for efficient eradication of the HIV-1 genome using the saCas9 / sgRNA system were optimized and tested with HIV-diploid relative quadruple Feasibility and efficiency of sgRNA excision with saCas9 in HIV-1 Tg26 transgenic mice and EcoHIV enhanced firefly luciferase (eLuc)-infected mice in 1 primary animal (Rabinovich, B.A. et al. (2008) Proc Natl AcadSci USA 105, 14342-14346; Potash, M.J. et al. Proc Natl Acad Sci USA 102, 3760-3765, doi:10.1073 / pnas.0500649102 (2005)). Furthermore, it was demonstrated that polyploid sgRNA / saCas9 delivered by an all-in-one AAV vector could be used as pre-exposure prophylaxis (PrEP) in vivo.
[0250] Materials and methods
[0251] Bioinformatics design of highly efficient gRNAs. Efficient gRNA design was performed using the B...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com