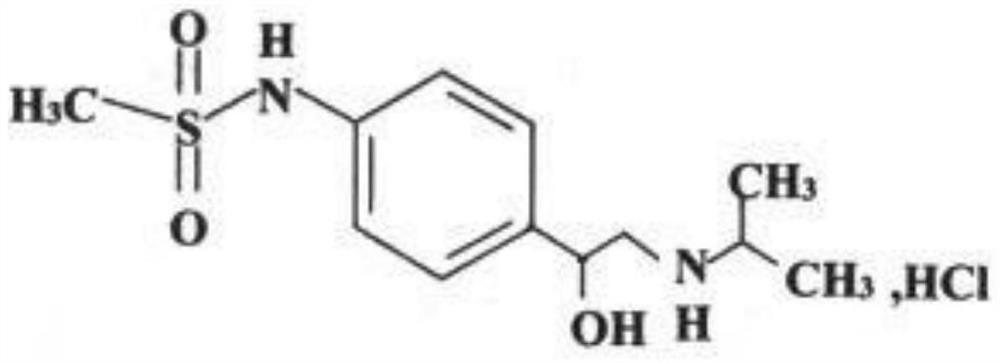
A kind of sotalol hydrochloride preparation
A technology of sotalol hydrochloride and hydrochloric acid, which is applied in the field of medicine, can solve the problems of untimely treatment of arrhythmia patients, poor dissolution rate and low dissolution rate of sotalol hydrochloride tablets, and facilitate industrialized large-scale production and improve drug production. Good curative effect and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
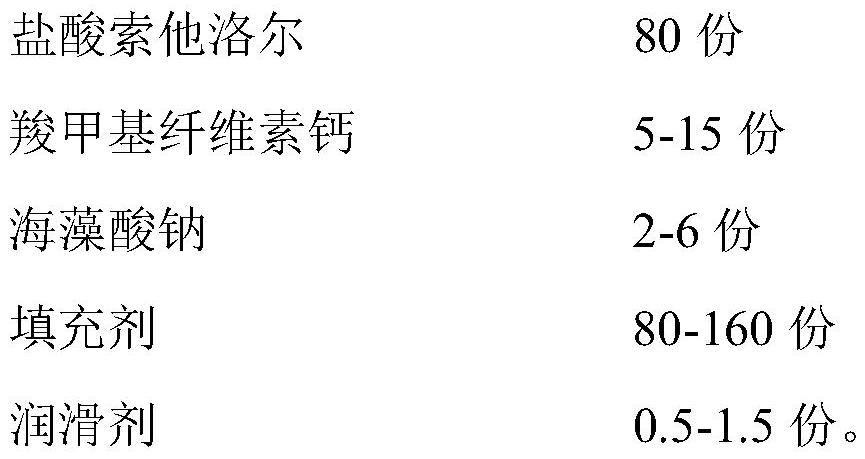
[0031] Prescription of 1000 tablets
[0032]
[0033] Preparation:
[0034] 1) Micronize 80g of sotalol hydrochloride and pass through a 100-mesh sieve, mix evenly with 10g of carmellose calcium, 4g of sodium alginate and 120g of filler;
[0035] 2) Mix the binder solution with the mixture in step 1) to make a soft material, pass through a 20-mesh sieve, and dry at 55-65°C for 4 hours;
[0036] 3) Mix the mixture in step 2) with 1.0 g of lubricant and press into tablets to make 1000 tablets.
[0037] 4) Mix hypromellose, povidone, and talcum powder, add purified water, stir for 0.5-1h, and make a suspension;
[0038] 5) Add the plain tablet prepared in step 3) into a multifunctional coating machine, and preheat to 60-65°C;
[0039] 6) Spray the suspension in step 1) evenly on the plain tablet through a peristaltic pump. During the spraying process, control the air inlet temperature to 65-75° C., stop coating after the weight gain is 2.5%, and let it cool to obtain.
Embodiment 2
[0041] 1) Micronize 80g of sotalol hydrochloride and pass through a 100-mesh sieve, mix evenly with 5g of carmellose calcium, 6g of sodium alginate and 120g of filler;
[0042] 2) Mix the binder solution with the mixture in step 1) to make a soft material, pass through a 20-mesh sieve, and dry at 55-65°C for 4 hours;
[0043] 3) Mix the mixture in step 2) with 1.0 g of lubricant and press into tablets to make 1000 tablets.
[0044] 4) Mix hypromellose, povidone, and talcum powder, add purified water, stir for 0.5-1h, and make a suspension;
[0045] 5) Add the plain tablet prepared in step 3) into a multifunctional coating machine, and preheat to 60-65°C;
[0046] 6) The suspension in step 1) is evenly coated on the plain tablet by spraying with a peristaltic pump. During the spraying process, the temperature of the air inlet is controlled to be 65-75° C., and the coating is stopped after the weight gain is 1.0%, and allowed to cool.
Embodiment 3
[0048] 1) Micronize 80g of sotalol hydrochloride and pass through a 100-mesh sieve, mix evenly with 15g of carmellose calcium, 2g of sodium alginate and 120g of filler;
[0049] 2) Mix the binder solution with the mixture in step 1) to make a soft material, pass through a 20-mesh sieve, and dry at 55-65°C for 4 hours;
[0050] 3) Mix the mixture in step 2) with 0.5 g of lubricant and press into tablets to make 1000 tablets.
[0051] 4) Mix hypromellose, povidone, and talcum powder, add purified water, stir for 0.5-1h, and make a suspension;
[0052] 5) Add the plain tablet prepared in step 3) into a multifunctional coating machine, and preheat to 60-65°C;
[0053] 6) Spray the suspension in step 1) evenly on the plain tablet through a peristaltic pump. During the spraying process, control the air inlet temperature to 65-75° C., stop coating after the weight gain is 4.5%, and let it cool to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com