Docetaxel target prodrug and application thereof on preventing colorectal carcinoma
A doene taxane and colon cancer technology, applied in the field of biomedicine, can solve the problems of poor selectivity of cytotoxic small molecule compounds, high systemic toxicity, and expanded application, and achieve good anti-colon cancer potential and good anti-colon cancer activity , high biocompatibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
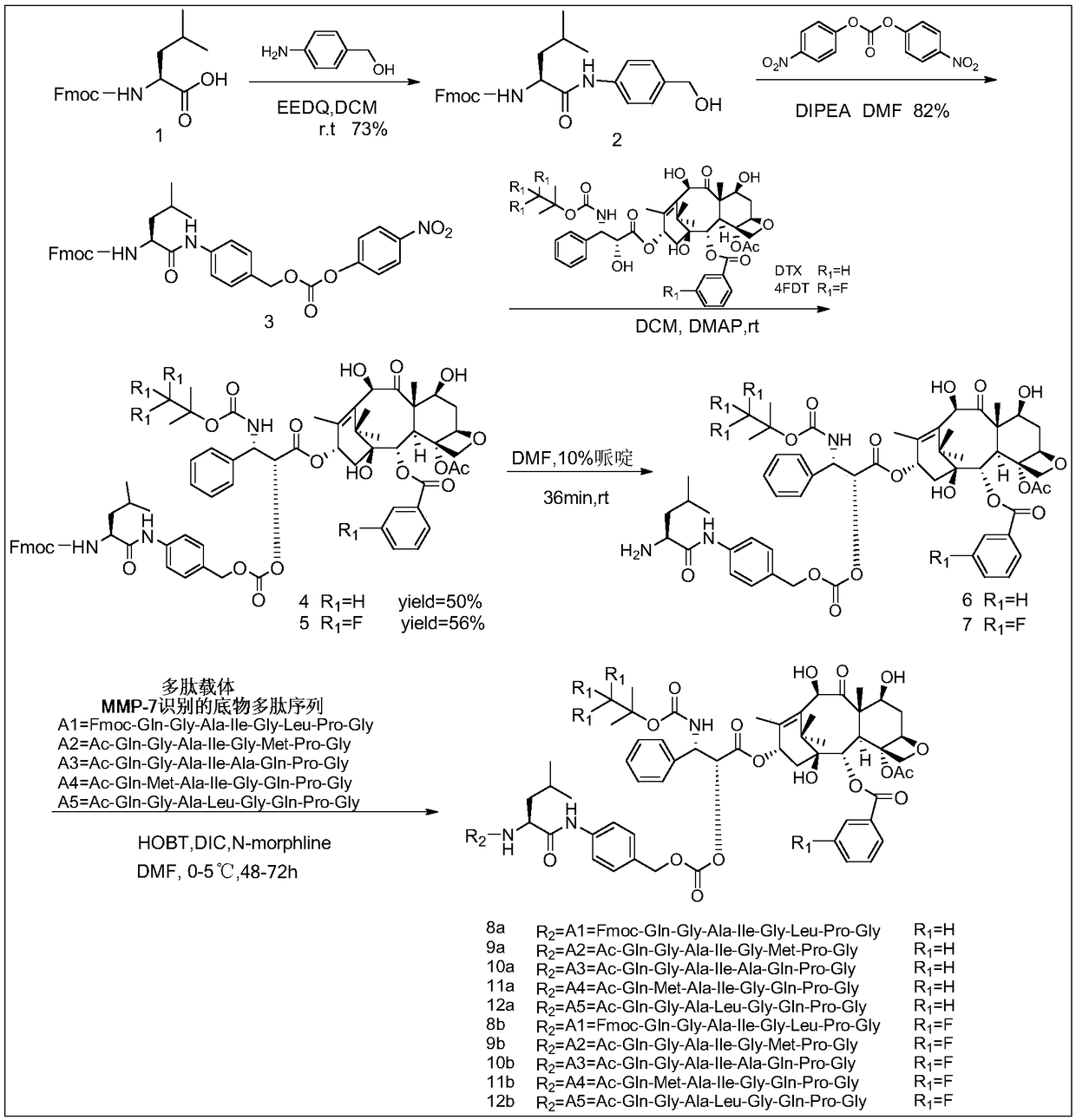
[0025] Example 1: Preparation of docetaxel or tetrafluorodcetaxel coupled with colon cancer targeting polypeptide A1-A5 prodrug via bridging group Leu-PABOH(a)
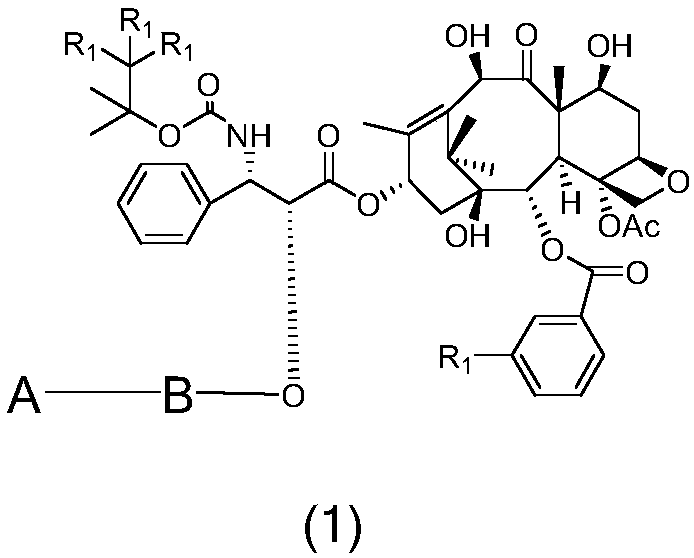
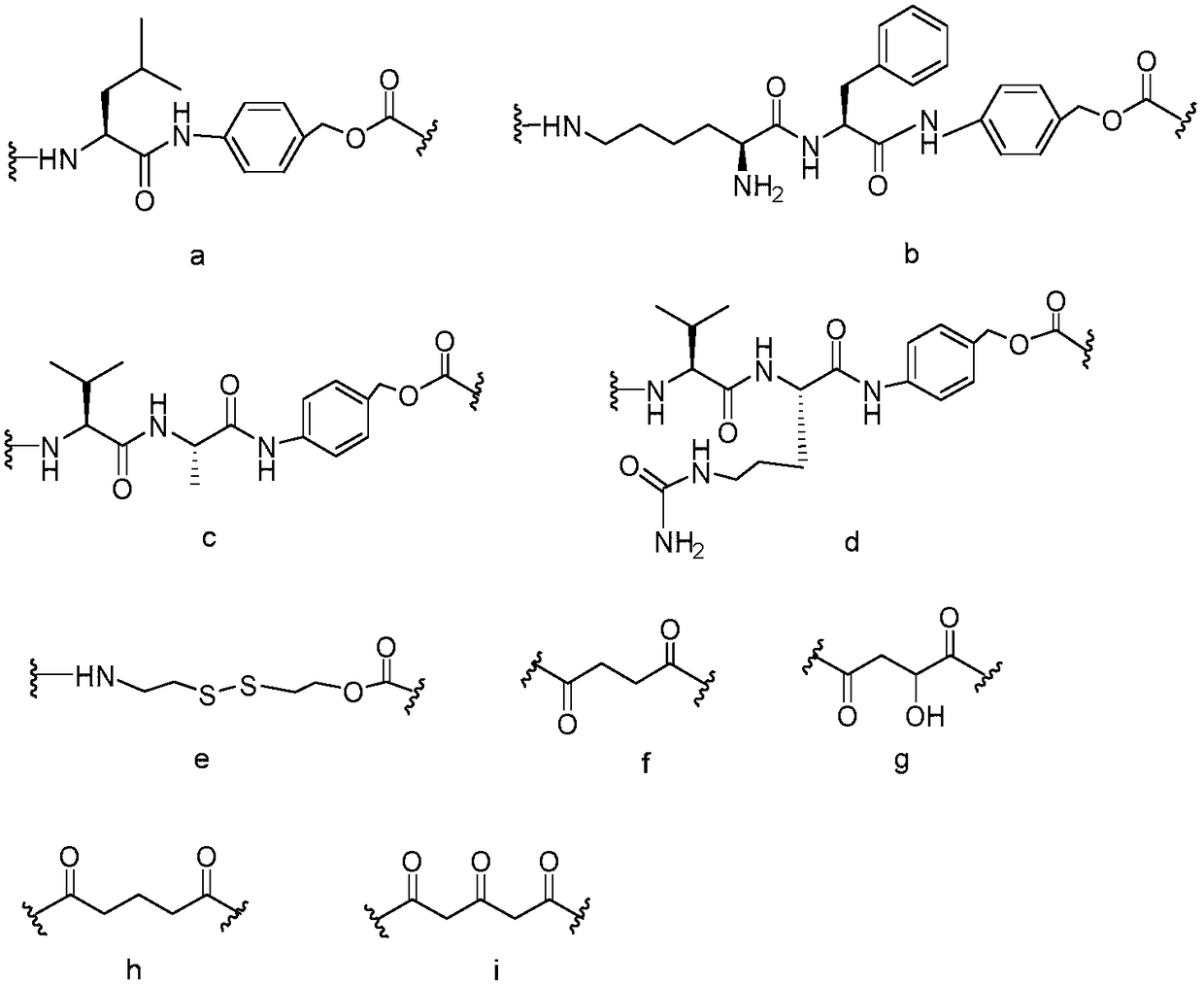
[0026] Taxane targeting prodrugs were prepared by coupling paclitaxel (DTX) or tetrafluorodcetaxel (4FDT) with colon cancer targeting polypeptides A1-A5, and the synthetic route was as follows: figure 1 shown, including the following steps:
[0027] 1) Synthesis and activation modification of bridging group Leu-PABOH
[0028]
[0029]References (Elsadek B, Graeser R, Esser N, et al. Development of a novelprodrug of paclitaxel that is cleaved by prostate-specific antigen: An invitro and in vivo evaluation study [J]. European Journal of Cancer, 2010, 46( 18): The method adopted in 3434-3444) completes the synthesis and activation modification of the bridging group Leu-PABOH through a two-step reaction. That is: in a 25mL single-necked bottle, add 1mmol of Fmoc-L-Leu (1), 2mmol of p-aminobenzyl alcohol (PABOH) and 2...
Embodiment 2
[0070] Example 2: Proliferation inhibitory activity of targeted anti-colon cancer prodrugs on human colon cancer cell lines HCT116 and SW620 in vitro and toxicity evaluation on normal colon cell line CCD18Co and kidney cell line HEK293
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com