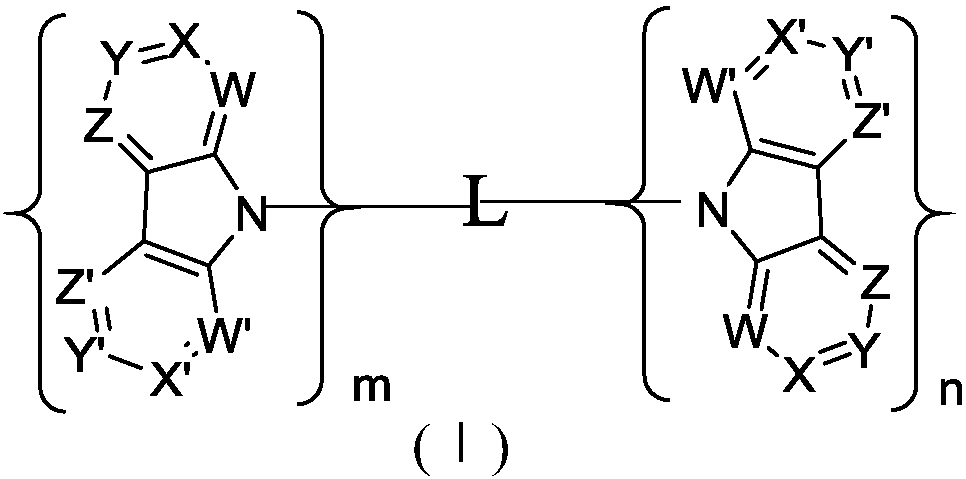
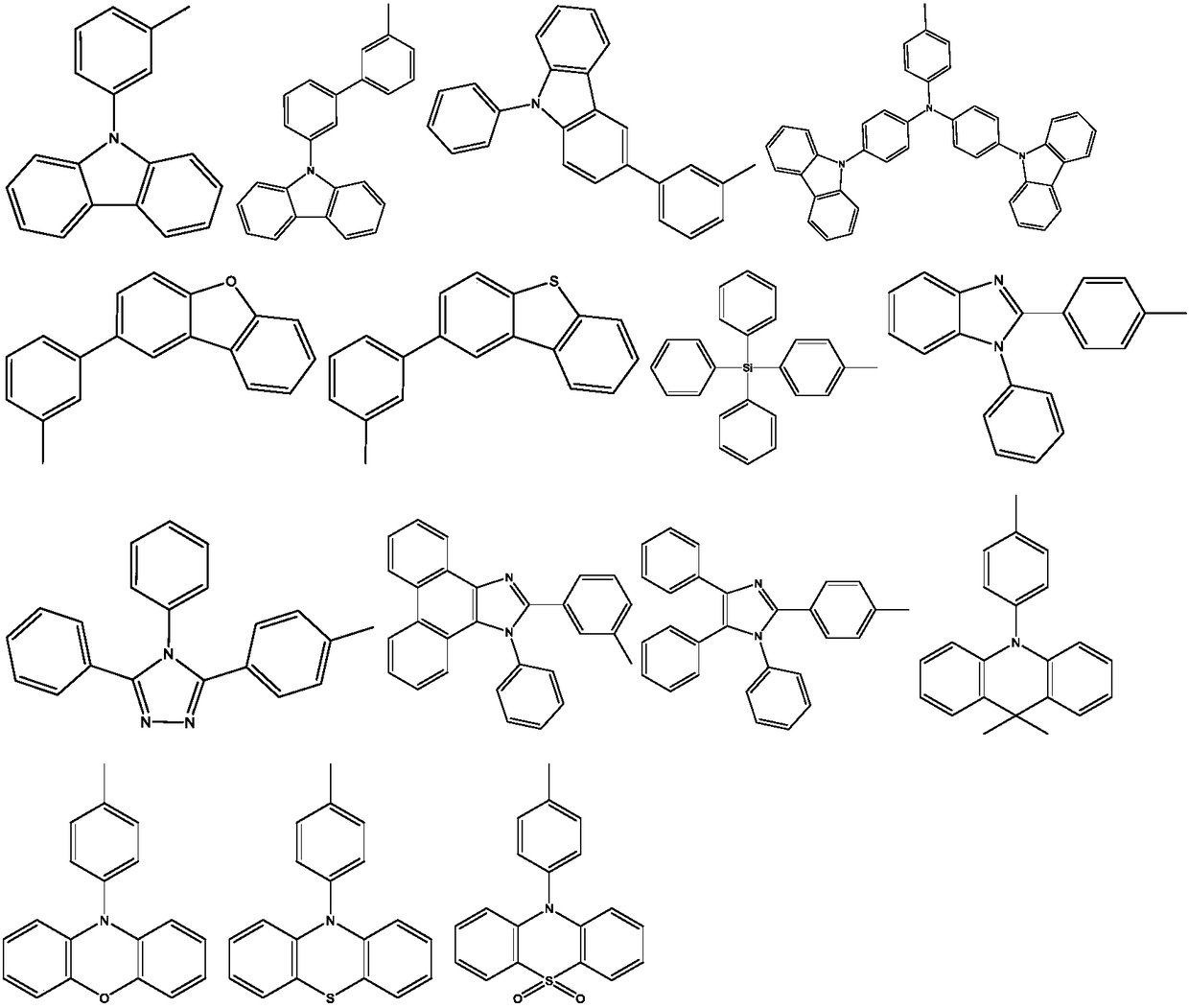
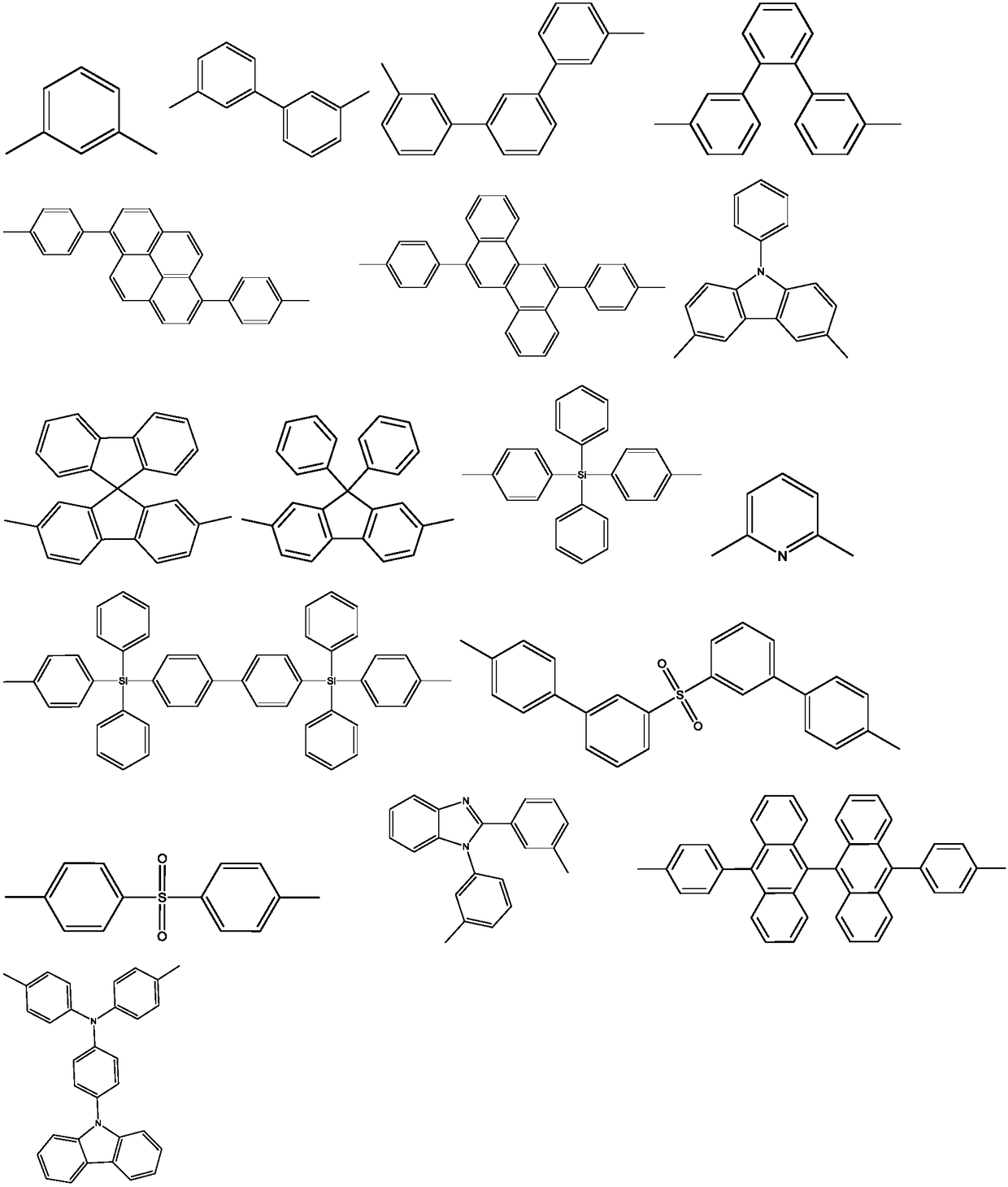
Diazcarbazole derivative, preparation method thereof, and application of diazcarbazole derivative as electroluminescent material
An azacarbazole and derivative technology, applied in the application field of organic optoelectronic materials, can solve problems such as low luminous efficiency, and achieve the effect of good carrier migration characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Embodiment 1, the preparation of compound 1
[0112]
[0113] Add 3-chloro-2-nitropyridine (5g, 31.63mmol), 3-pyridineboronic acid (3g, 37.75mmol) into a 500ml single-necked flask, dissolve 50ml of 2M aqueous potassium carbonate in 50ml of ethanol, and dissolve in a solvent of 100ml of toluene . in N 2 Under the protection conditions, adding PdCl 2 (PPh 3 ) 2 (0.75 g, 0.98 mmol). The temperature was raised slowly to 100°C, and the mixture was reacted under reflux for 24h. After cooling, the liquid was separated, the organic layer was rotary evaporated, and the column was passed through petroleum ether and ethyl acetate (1.5:1) to obtain 4.5 g of a light yellow solid with a yield of 65%. MS(APCI)m / z calcd for C 10 h 7 N 3 o 2 :201.20,found[M + ]:201.89.
Embodiment 2
[0114] Embodiment 2, the preparation of compound 2
[0115]
[0116] Add 3-(2-nitropyridin-3-yl)pyridine (4.06g, 20.25mmol) and triphenylphosphine (13.3g, 50.65mmol) solvent in 100ml o-dichlorobenzene solvent in a single-necked flask of 250ml . in N 2Under the condition of protection, the temperature was raised to 180°C, and the reaction was refluxed for 24h. After the reaction was completed, distill under reduced pressure, concentrate the reaction solvent to 40ml, and pass through the column with dichloromethane and methanol (15:1) to obtain 2g of white solid with a yield of 60%. MS(APCI)m / z calcd for C 10 h 7 N 3 : 169.20, Found[M + ]:169.69.
Embodiment 3
[0117] Embodiment 3, the preparation of compound 3
[0118]
[0119] Add compound 2 (1g, 6mmol) in the single-necked flask of 250ml, o-bromoiodobenzene (2g, 7.2mmol), copper powder (1.2g, 18mmol), K 2 CO 3 (2.5g, 18mmol) was dissolved in 100ml of dry DMF solvent. in N 2 Under the conditions of protection, the temperature was raised to 150°C, and the reaction was carried out for 48h. After the reaction was terminated, it was cooled to room temperature, extracted, spin-dried, and passed through the column to obtain a light yellow liquid with a yield of 70%. MS(APCI) m / z calcd for C 16 h 10 BrN 3 : 323.00, Found[M + ]:324.04.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com