Metal organic complex as well as preparation method and application thereof
A compound and reaction technology, applied in the field of metal-organic complexes and their preparation, can solve the problems of weakening luminescence and increasing non-radiative transitions, and achieve the effect of high electroluminescence efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
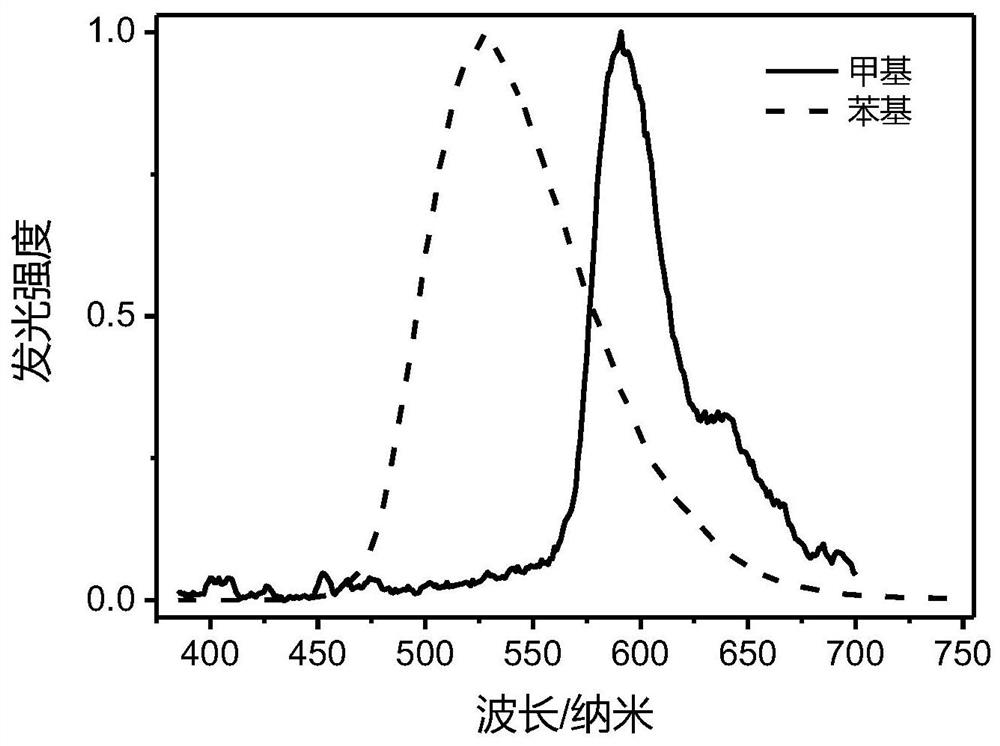
[0048] Firstly, methyl-substituted carbene compound A2 and phenyl-substituted carbene compound E2 are prepared.
[0049] The preparation process of A2 is: add 2,3-dichloropyrazine (0.26g, 4.10mmol), methylamine (33% aqueous solution, 14ml) into a 25ml reaction kettle after stirring and mixing, and place the reaction kettle in an oven Reacted at 140°C for 10 hours, placed in a refrigerator for crystallization, and obtained 2,3-dimethylaminopyrazine as a yellow-green needle-like solid (2.51 g, yield: 90%).
[0050] 2,3-Dimethylaminopyrazine (2.51g, 18mmol), triethyl orthoformate (10ml) and 37% concentrated hydrochloric acid (1ml) were mixed and refluxed overnight at 120°C. After the reaction was completed, cool down to room temperature and add 50ml of ether , the product was precipitated out. The solid was collected by filtration, decolorized and recrystallized with 20ml of ethanol and activated carbon. After filtration, the filtrate was spin-dried and the solid was recrystall...
Embodiment 2
[0089] By grinding and other means, the light-emitting mechanism of the compound can be changed. Grinding the metal complex A2 can obviously observe the change of luminescence, and its luminescence spectrum is as follows Figure 6 shown by Figure 6 It can be known that its emission spectrum produces a new emission peak at 540nm after grinding at low temperature. The photophysical data of Compound A2 are shown in Table 3 below. The data in Table 3 also show that the lifetime of Compound A2 has changed significantly with grinding, from the original 700 microseconds to about 1 microsecond, and the luminescence mechanism changed from the original L 'LCT-induced room temperature phosphorescence is changed to MLCT or MMLCT-induced phosphorescence emission with the participation of metal d orbitals. Due to the participation of metals, the spin-orbit coupling is enhanced, so the intersystem crossing rate is accelerated, resulting in a significant reduction in luminescence lifetime. ...
Embodiment 3
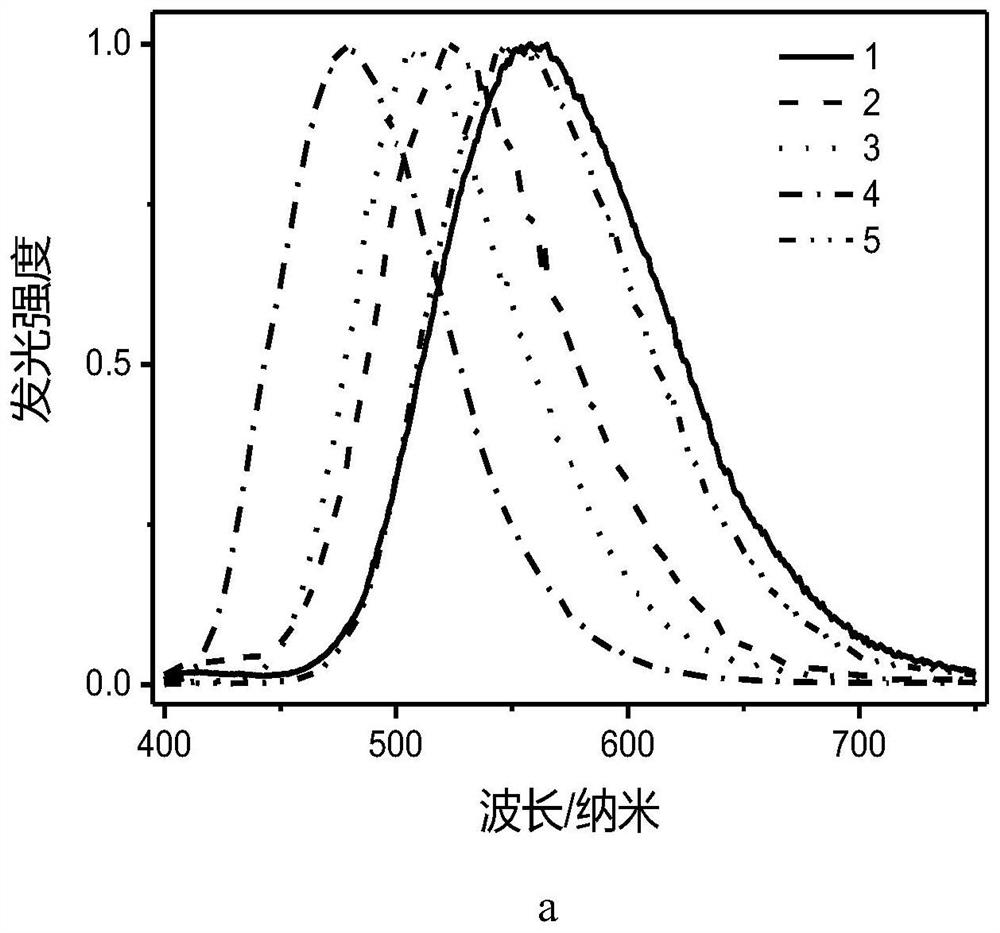
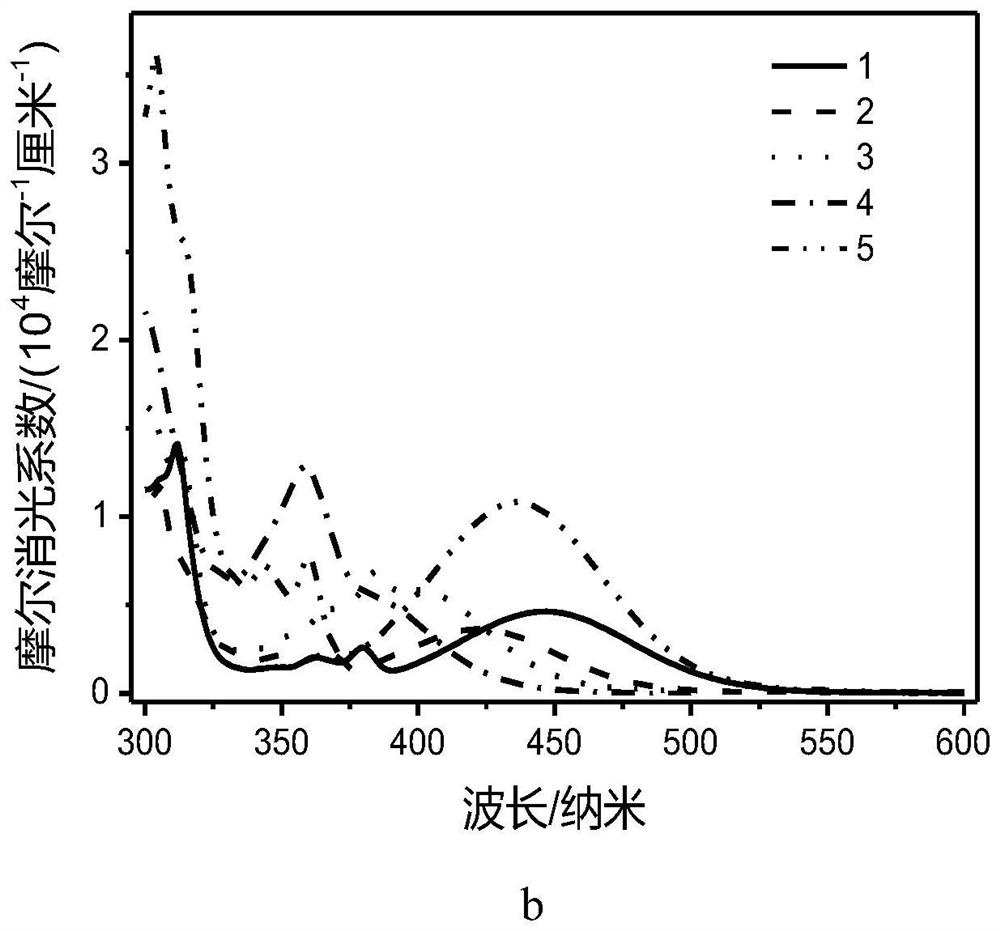
[0093] The compound E1-5 prepared in Example 1 realizes the construction of a donor-acceptor structure, so that the energy range between the singlet state and the triplet state of this type of compound is less than 0.1eV, thereby realizing the triplet excitons through reverse Intersystem crossing back to the singlet excitons, and then return to the ground state through the singlet state through radiative transition, accompanied by delayed fluorescence emission, because of the good use of the triplet excitons, and the short lifetime can effectively Reduce the roll-off efficiency, so the organometallic complex compound E1-5 has TADF properties, and its properties show that the luminescence lifetime is significantly increased at low temperatures, such as Figure 4 shown. Figure 4 For compound E1-5, the data of the tested life span as a function of temperature can be well fitted to satisfy the formula And it can well prove that the obtained compound E1-5 does have TADF, so it c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com