Application of sphingolipid inhibitor for preparing medicine of inhibiting iron overload disease
A technology of iron overload and inhibitor, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
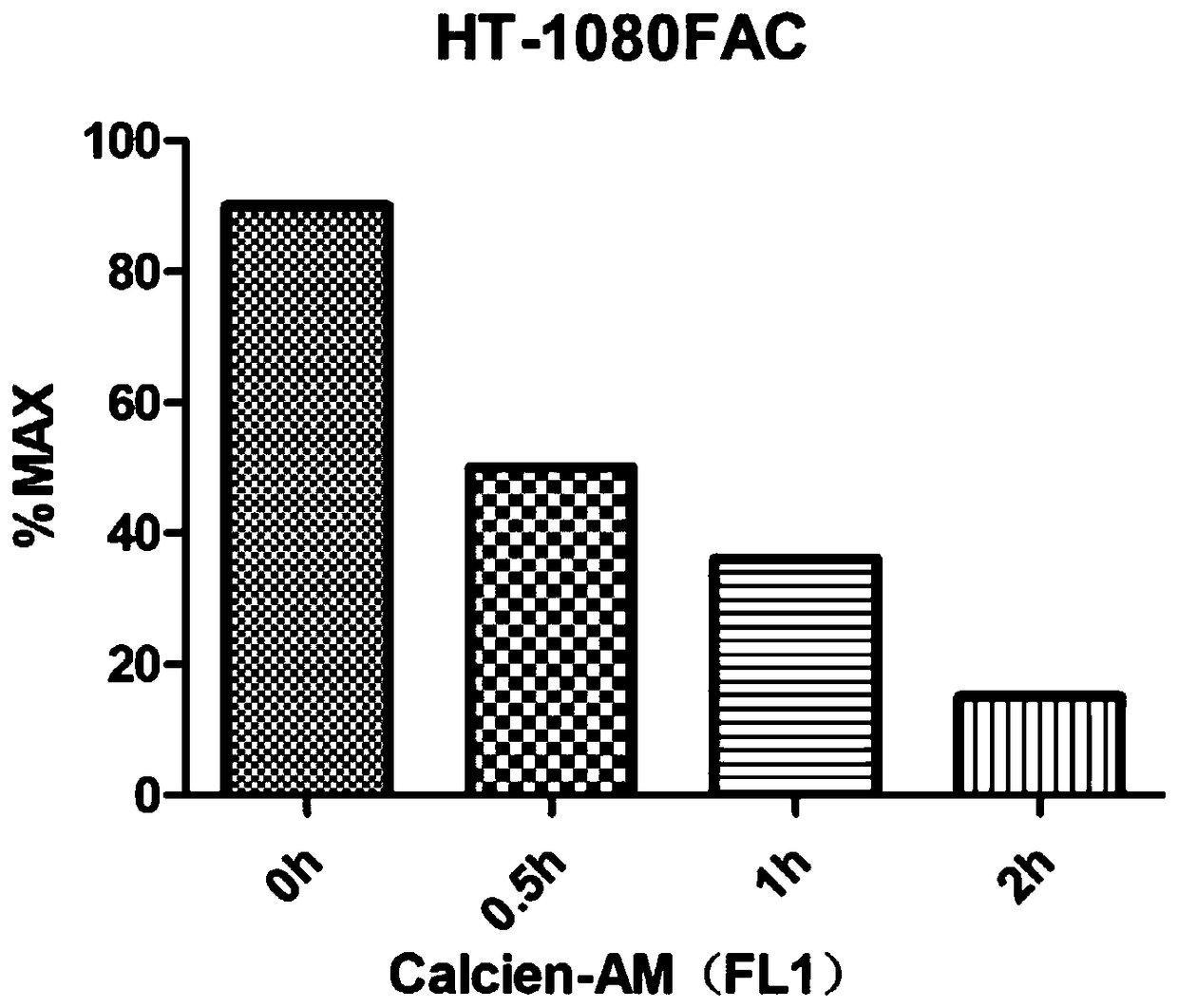
[0054] Example 1: Intracellular iron concentration measurement.
[0055] 1) The day before the test, the human fibroblastosarcoma cancer cells that had grown to 80% to 90% were digested and centrifuged, and mixed with 2×10 cells per well. 5 The number of cells, inoculated into 6-well cell culture plate, and the cells were grown for 24 hours;
[0056] 2) Treat human fibroblastosarcoma cancer cells with medium containing 5 mM ferric ammonium citrate (calculated as iron) for 0 hour, 0.5 hour, 1 hour, and 2 hours;
[0057] 3) After the treatment time is up, discard the culture medium in the six-well plate, wash it twice with PBS gently, add 1 mL PBS containing 100nM Calcein-AM, and stain in the cell culture incubator for 15 minutes;
[0058] 4) After the staining time is up, add 600 μL Accutase cell digestion solution to each well to digest the cells, add 2 mL PBS to collect the cells into a centrifuge tube, centrifuge at 2500 rpm for 5 minutes, discard the supernatant and add 50...
Embodiment 2
[0060] Embodiment 2: Optical microscope observation and photographing.
[0061] 1) Cells grown to 80%-90% confluence were digested and centrifuged, and 2×10 5 The number of cells, inoculated into 6-well cell culture plate, and the cells were grown for 24 hours;
[0062] 2) Human fibroblastosarcoma cancer cells were treated with culture solution containing 0mM or 5mM ferric ammonium citrate (calculated as iron) for 24 hours
[0063] 3) After the treatment time, observe the cell morphology under a LEICA DCF295 inverted microscope, and select three random areas in the field of view to take pictures. Each trial was performed three independent manipulations. The result is as figure 2 Shown in B.
Embodiment 3
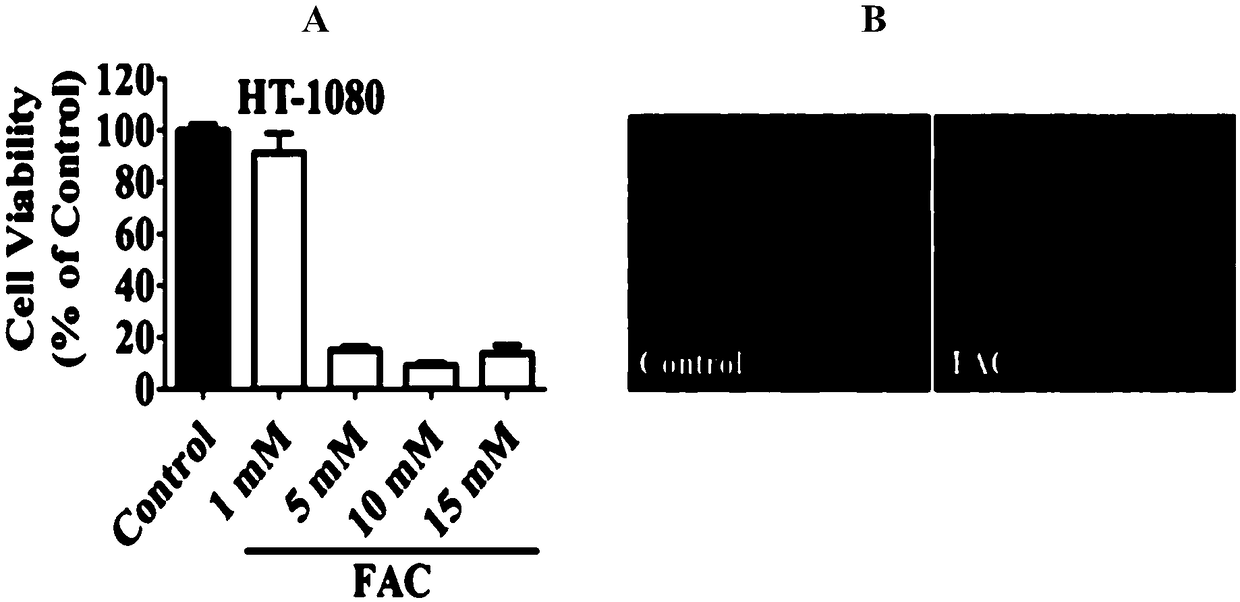
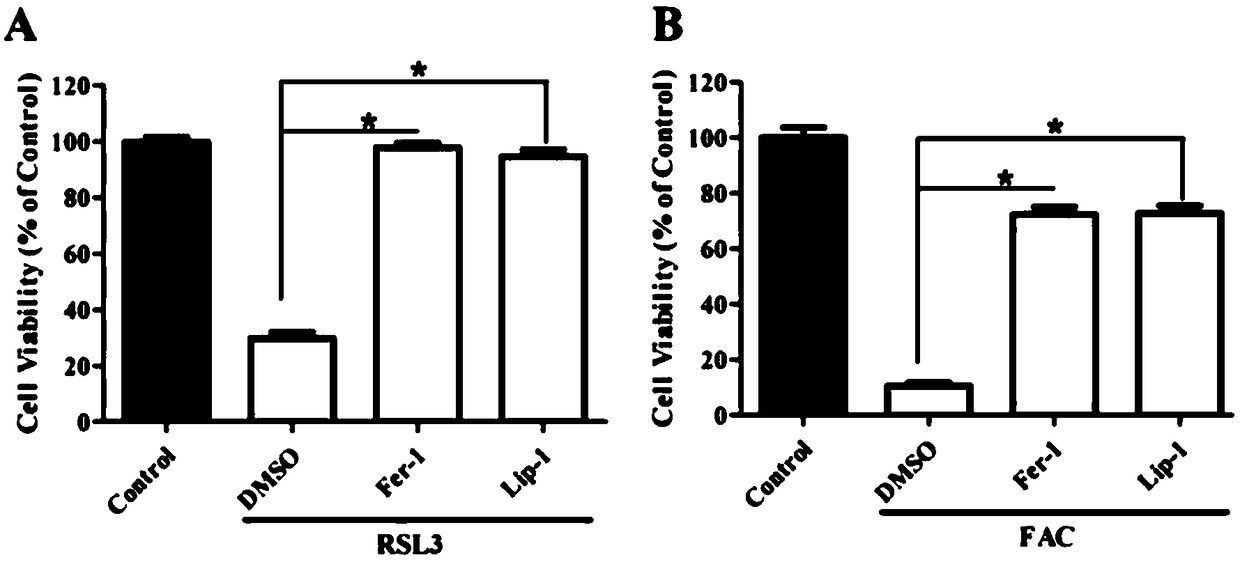
[0064] Example 3: Detection of cell activity.
[0065] 1) Digest and centrifuge the human fibroblastosarcoma cancer cells grown to 80%-90% fusion, 1×10 per well 4 The number of cells, each inoculated to a 96-well cell culture plate, cultivated for 24 hours;
[0066] 2) ①Add the control to the 96-well plate containing the cells, and culture the medium containing 1mM, 5mM, 10mM, 15mM FAC (calculated as iron) for 24 hours; ②Add the control (double distilled water) to the cells in the 96-well plate , 5mM FAC, 5mM FAC+10μM Fer-1, 5mM FAC+10μM Lip-1 medium to treat cells for 16 hours; ③Add control (DMSO), 2μM RSL3, 2μM RSL3+10μM Fer-1 to 96-well plate cells , 2μM RSL3+10μM Lip-1 medium for 8 hours; ④ medium containing control, 5mM FAC, 5mM FAC+100μM trolox, 5mM FAC+10μM U0126 medium for 24 hours; each treatment set 6 replicates;
[0067] 3) At 37°C, 5% CO 2 Conditioned cell culture incubator;
[0068] 4) Carefully pipette the treatment solution in the 96-well plate, and add the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com