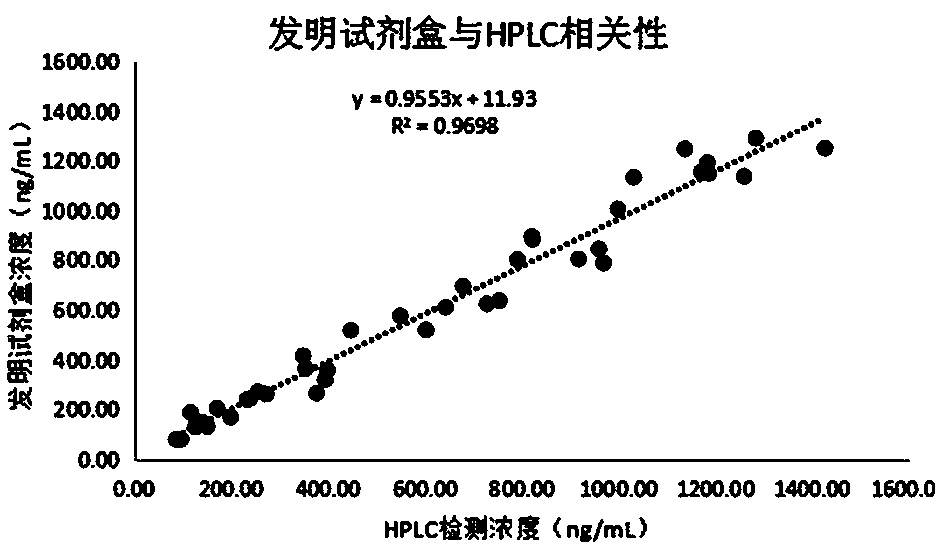
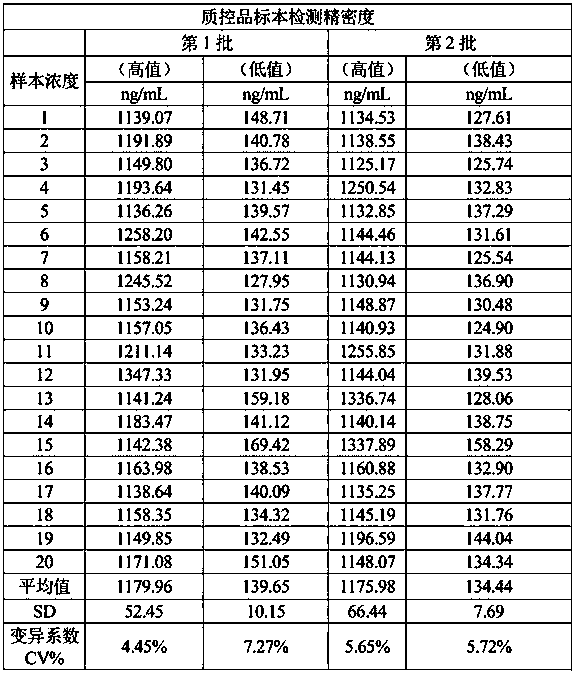
Luminescence immunoassay kit for metanephrine
A metanephrine, detection kit technology, applied in chemiluminescence/bioluminescence, measurement device, analysis by chemical reaction of materials, etc., can solve the problem of time-consuming HPLC and the lack of accuracy of enzyme-linked immunoassay To solve the problems of high reliability and reproducibility, and high cost, to achieve the effect of high sensitivity and precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of methoxyepinephrine luminescent immunoassay kit
[0032] 1. Preparation of solid phase carrier material
[0033] Preparation of magnetic microsphere suspension: firstly, wash the selected magnetic microsphere stock solution with 10 times the stock solution volume of PBS buffer for 2 to 5 times, then activate it with EDC, NHS or glutaraldehyde, and the activated magnetic microspheres Coat the antibody with a concentration of 5-40 μg / mL by any method of chemical connection. After the coated magnetic microspheres are washed and sealed with a sealing solution, they are fixed to volume and packaged, and stored at 2-8 °C. spare.
[0034] This method can be used to prepare a, magnetic particle-linked second antibody, b, magnetic particle-linked anti-EITC antibody, c, magnetic particle suspension of magnetic particle-linked anti-methoxyadrenaline antibody.
[0035] 2. Preparation of avidin-linked tracer solution
[0036] First, according to the formul...
Embodiment 2
[0048] Embodiment 2 The usage method of kit of the present invention
[0049] 1. Sample pretreatment: Take 10-50 μl of urine test sample into a 6 mL glass bottle, add 100-300 μl of acidification solution, acidify in a water bath (60-100°C) for 0.5-2 h, cool to room temperature, add 20- 100 μl of acylating agent was shaken on a shaker for 15-30 min, transferred to a cuvette, and detected using an AutoLumo automatic detection analyzer.
[0050] 2. Detection: Take the kit consisting of avidin-HRP solution, methoxyepinephrine antibody solution, common conventional substrate, and cleaning solution as an example: add the processed calibrator and sample into the cuvette respectively, add The sample volume was 50 μl / well. Add 20 μl of magnetic particle suspension, 50 μl of sample, and 50 μl of antibody solution to each well, mix well and incubate at 37°C for 15 minutes, and wash with washing solution 6 times. Add 100 μl of enzyme conjugate to each well, mix well and incubate at 37 °...
Embodiment 3
[0051] Embodiment 3 Performance evaluation of the kit of the present invention
[0052] 1. Sensitivity detection
[0053] Limit of Blank (LOB): 5 blank clinical samples with a value close to 0, each sample was repeated 3 times for a total of 4 days, and 60 data with non-negative results were obtained;
[0054] Line of Detection (LOD): After the LOB is determined, collect 5 clinical samples with a low value of 1 to 4 times the LOB, repeat 3 times for each sample, and do a total of 4 days to obtain 60 data;
[0055] Functional Sensitivity (FS): Using the data in the LOD experiment, 5 concentration samples were tested 3 times a day for a total of 4 days, and each sample obtained 12 results, and the mean, SD and CV% of each sample were calculated, and the nearest 20 The concentration of % is the functional sensitivity; the specific data are shown in Table 1.
[0056] Table 1 Sensitivity detection of the kit of the present invention
[0057]
[0058] The results in Table 1 sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com