HCV NS3 antibody detection method based on MIX antigen coating
A detection method and antigen technology, applied in the field of biotechnology diagnosis, can solve the problem of not being able to detect antibodies as effectively, and achieve the effect of reducing missed detection and improving the detection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] HCV NS3 antibody detection program optimization
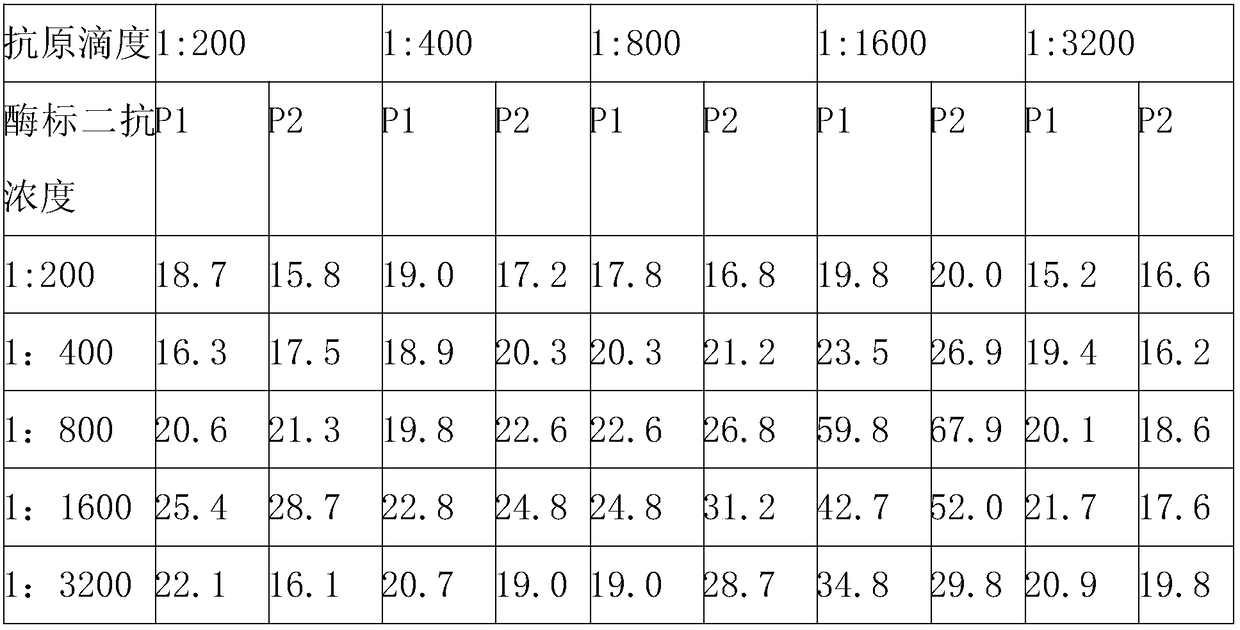
[0045] 1. Selection of the optimal dilution of NS3MIX coating antigen and enzyme-labeled antibody
[0046] 1. Method: Use the square array titration method to coat the purified MIX antigen containing HCV NS3 (type 1) and NS3 (type 6) in multiple dilutions, and at the same time, detect the HRP-labeled goat anti-human IgG For doubling dilution, the dilution ratios of both are from 1:200 to 1:3200, and 2 negative samples and 2 positive samples are detected respectively, and the sample volume is 1 μl, and a blank control well is set up. The specific operation steps are as follows, Finally select the positive serum OD 450 Value and Negative Serum OD 450 The dilution with the highest ratio of values is the optimal dilution.
[0047] 2. Specific steps: Dilute the NS3MIX antigen with 0.01mol / L PBS (pH 7.2) and coat the microplate, 100μl / well, put it in a refrigerator at 4°C overnight; wash 3 times with 1×PBST solution the n...
Embodiment 2
[0078] Repeatability and Stability Test of HCV NS3 Antibody Detection
[0079] 1. Repetitive test
[0080]1. Method: Get 3 positive specimens, measure 10 times simultaneously, calculate intra-assay coefficient of variation (CV), measure once a day for the same 3 specimens and measure continuously for 5 days, calculate inter-assay coefficient of variation.
[0081] 2. Specific steps: the detailed operation steps and methods are the same as items 1 and 2 in Example 1.
[0082] 3. Results: 3 positive serum samples were measured 10 times at the same time period, and the intra-assay coefficient of variation was calculated; the same 3 positive serum samples were continuously measured 5 times, once a day, and the inter-assay coefficient of variation was calculated; the result coefficients of variation were all less than 10%, showing that the repeatability of the method is good (shown in the table below).
[0083] Repeatability test results (CV)
[0084]
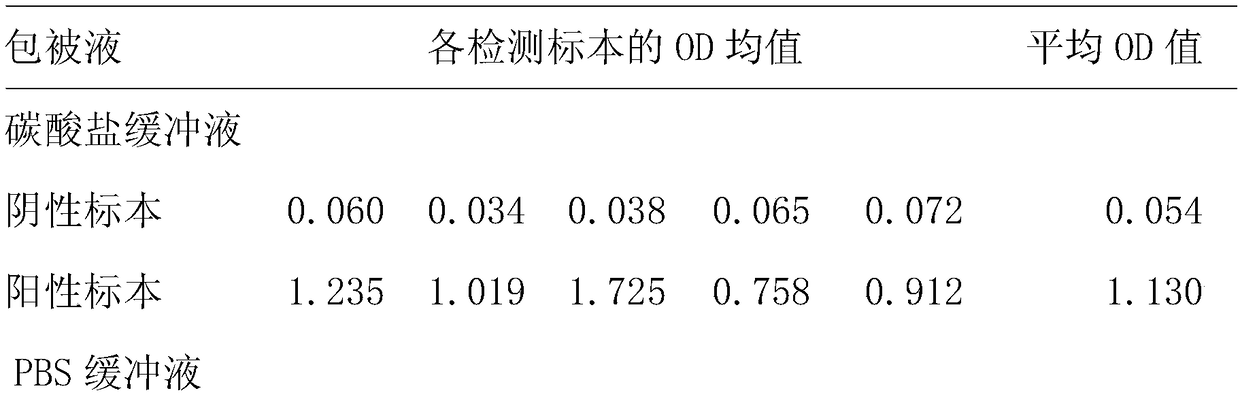
[0085] 2. Stability te...
Embodiment 3
[0093] Sensitivity and Specificity Test of HCV NS3 Antibody Detection
[0094] 1. Methods and steps
[0095] (1) Detect 90 anti-HCV antibody positive sera.
[0096] (2) HCV antigen neutralization test: take 10 anti-HCV antibody positive sera and add HCV recombinant MIX antigen respectively, and incubate at 37°C for 1 hour; take the same 10 positive sera as the control group, but without adding MIX antigen, do the same Incubate at 37°C for 1 hour; then test the samples with the established detection method respectively.
[0097] (3) 6 anti-HAV antibody positive, 9 anti-HBV antibody positive, and 20 anti-HEV antibody positive serum samples were tested respectively.
[0098] (4) Detect 200 serum samples from healthy physical examination and 20 negative quality control serum samples from clinical examination centers.
[0099] 2. Results
[0100] (1) 90 anti-HCV antibody positive serum specimens were tested, and as a result, 65 were positive for HCV NS3 antibody, and the positi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com