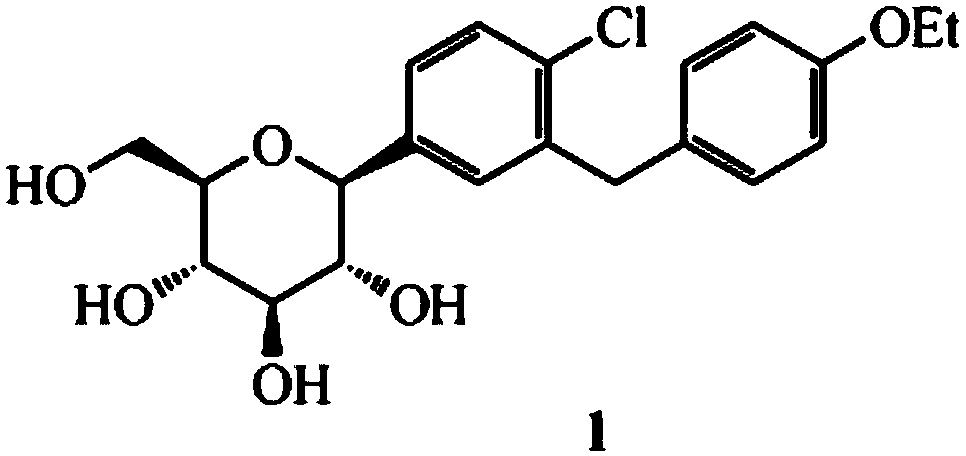
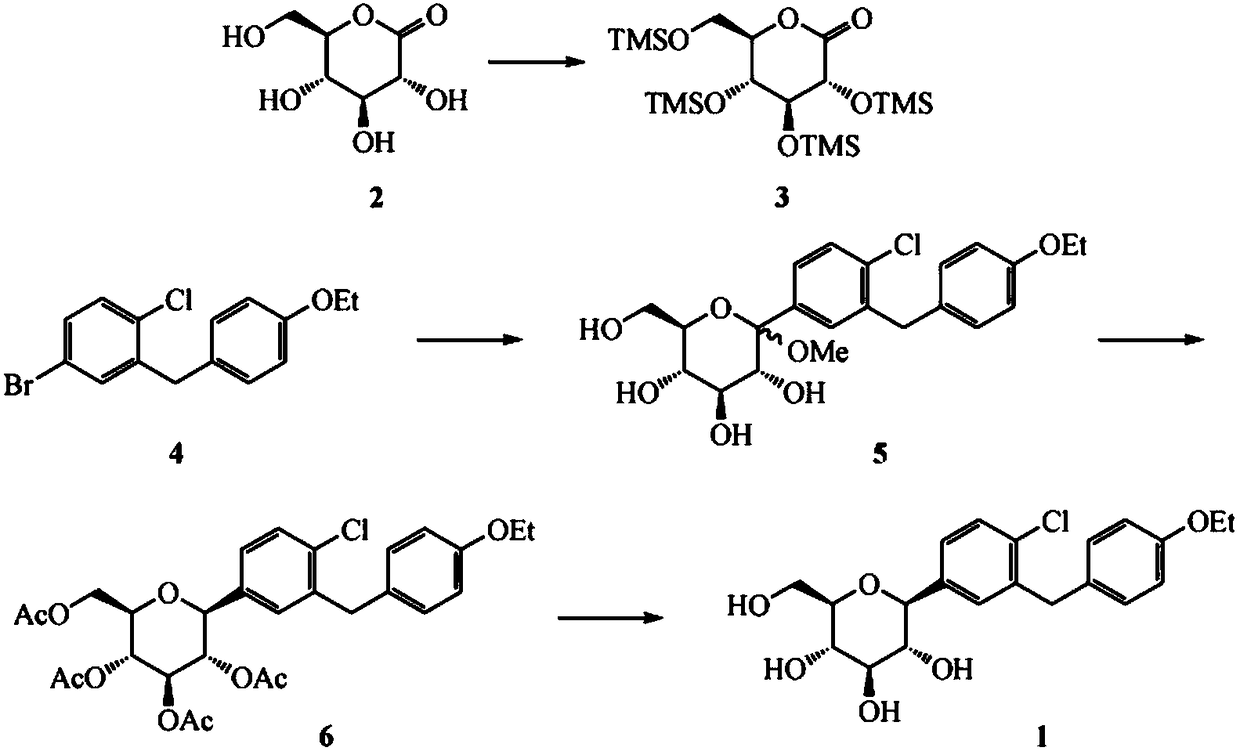
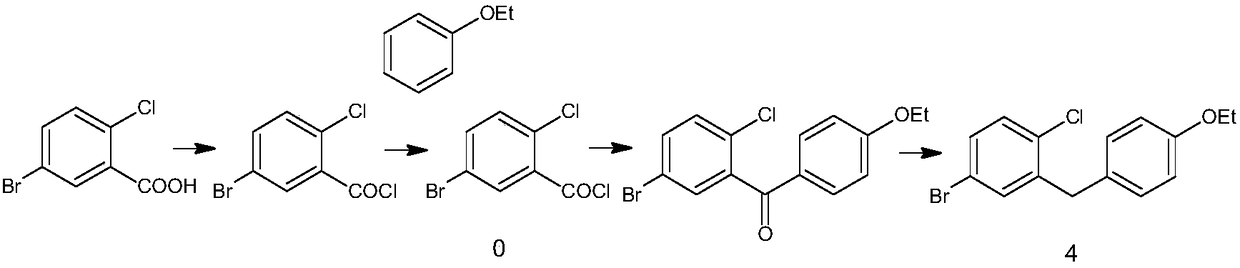
Synthesis method of dapagliflozin midbody 5-bromine-2-chloro-benzoyl chloride
The technology of a kind of chlorobenzoyl chloride and synthesis method is applied in the field of synthesis of dapagliflozin intermediate 5-bromo-2-chlorobenzoyl chloride, which can solve the problems of three wastes pollution and unsafe operation, and achieve less side reactions, The effect of safe operation and low process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthetic method of 5-bromo-2-chlorobenzoyl chloride comprises the following steps:
[0033] 1) Mix monomethyl oxalyl chloride and 1,1,1-trichloroethane, add the catalyst, stir and pass in argon, control the pressure to 6 atmospheres, control the temperature to 125°C, maintain for 1.5h, and then First add the solution of 4-bromochlorobenzene and chloroform dropwise. After the addition is complete, the temperature of the system is raised to 165° C., and the pressure is raised to 8 atmospheres to continue the reaction for 14 hours.
[0034] The catalyst is obtained by the following method: grind 4A molecular sieves, pass through 350 meshes, soak for 4 days in 12% copper sulfate aqueous solution, filter, and activate at 400°C; the weight ratio of molecular sieves to copper sulfate aqueous solution is 1:7.2.
[0035] The molar ratio of 4-bromochlorobenzene to monomethyl oxalyl chloride is 1:1.12; the dosage ratio of monomethyl oxalyl chloride to 1,1,1-trichloroethane i...
Embodiment 2
[0038] The synthetic method of 5-bromo-2-chlorobenzoyl chloride comprises the following steps:
[0039] 1) Mix allyl oxalyl chloride monoallyl with carbon tetrachloride, add the catalyst, stir and feed nitrogen, control the pressure to 4 atmospheres, control the temperature to 110 ° C, keep it for 1 hour, and then add 4-bromochloro After adding the solution of benzene and 1,2-dichloroethane, the temperature of the system was raised to 150°C, and the pressure was raised to 5 atmospheres to continue the reaction. 10 End.
[0040] The catalyst is obtained by the following method: grind 5A molecular sieves, pass through 200 meshes, soak in 10% copper sulfate aqueous solution for 2 days, filter, and bake and activate at 300°C; the weight ratio of molecular sieves to copper sulfate aqueous solution is 1:5.
[0041] The molar ratio of 4-bromochlorobenzene to monoallyl oxalyl chloride is 1:1.05; the dosage ratio of monoallyl oxalyl chloride to carbon tetrachloride is 1g:8ml; 4-bromoc...
Embodiment 3
[0044] The synthetic method of 5-bromo-2-chlorobenzoyl chloride comprises the following steps:
[0045] 1) Mix ethyl oxalyl chloride and 1,1,1-trichloroethane evenly, add catalyst, stir and pass protective gas, control the pressure to 7 atmospheres, control the temperature to 136°C, keep it for 2 hours, and then drop it first After adding a solution of 4-bromochlorobenzene and chloroform, the temperature of the system was raised to 170° C., and the pressure was increased to 9 atmospheres to continue the reaction for 16 hours.
[0046] The catalyst is obtained by the following method: grind the 4A type molecular sieve, pass through 400 meshes, soak in 15% copper sulfate aqueous solution for 5 days, filter, and bake and activate at 450°C; the weight ratio of molecular sieve to copper sulfate aqueous solution is 1:8.
[0047] The molar ratio of 4-bromochlorobenzene to ethyl oxalyl chloride is 1:1.15; the amount ratio of ethyl oxalyl chloride to 1,1,1-trichloroethane is 1g:12ml; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com