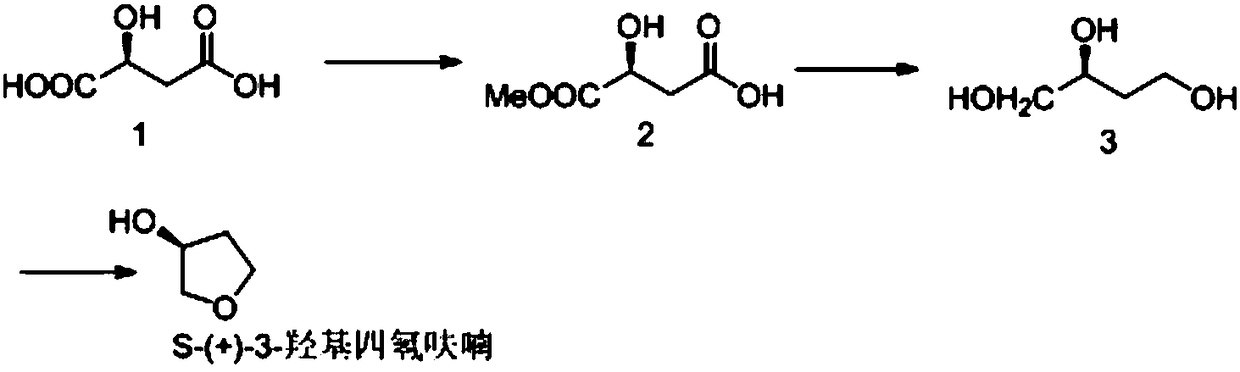
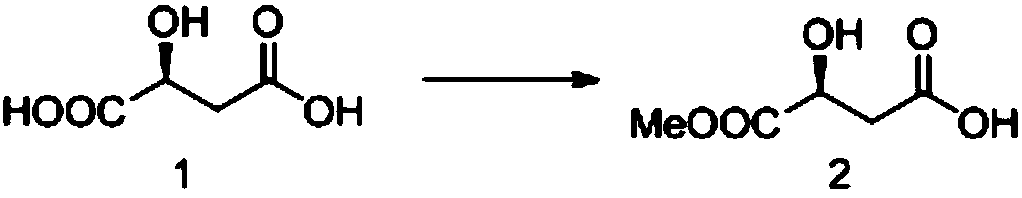Chemical synthesis method of S-(+)-3-hydroxytetrahydrofuran
A technology of hydroxytetrahydrofuran and chemical synthesis, applied in the directions of organic chemistry methods, organic chemistry, etc., can solve the problems of complicated operation, low yield, unguaranteed purity, etc., and achieve the effect of high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation of embodiment 1 S-dimethyl malate (compound 2)
[0018]
[0019] Add L-malic acid (compound 1, 40.3g, 0.35mol) and anhydrous MeOH (120mL) to the reaction flask, cool to 0°C, add thionyl chloride (49mL, 0.56mol) dropwise, after the addition is complete , the reaction naturally rose to room temperature, and reacted overnight. After the reaction was completed, the reaction solution was concentrated under reduced pressure, dichloromethane (120 mL) was added to dissolve the residue, and washed with saturated sodium carbonate until neutral. The organic phase was dried, filtered and concentrated to obtain compound 2 (43 g).
Embodiment 2
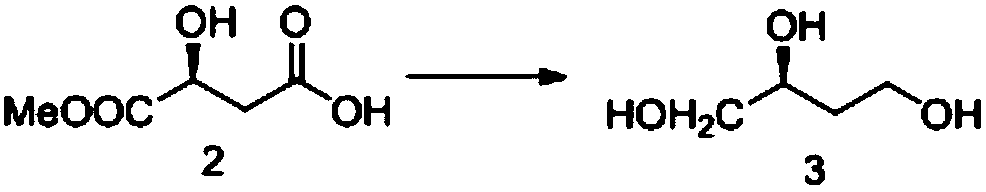
[0020] Example 2 Preparation of S-1,2,4 butanetriol (compound 3)
[0021]
[0022] Compound 2 (8.6 g, 0.05 mol) was dissolved in THF (100 mL), under nitrogen protection, 0.23 g of sodium carbonate was added, and sodium borohydride (7 g, 0.21 mol) was added in batches at a temperature lower than 40°C, and stirred for 1 h. Heated to reflux for 10h. Concentrate to remove the solvent, add concentrated hydrochloric acid to adjust the pH of the solution to neutral, extract with 200mL ethyl acetate, concentrate compound 3 (4g), and directly put the crude product into the next reaction without purification.
Embodiment 3
[0023] Example 3 Preparation of S-(+)-3-hydroxytetrahydrofuran
[0024]
[0025] Take the crude product compound 3 (15.9 g, 0.15 mol) obtained in the previous step reaction in a reaction flask. Add p-toluenesulfonic acid monohydrate (0.5 g, 2.7 mmol)). Stir, reduce pressure, and heat to dissolve p-toluenesulfonic acid. Gradually raise the temperature to 90°C, a colorless liquid drops out at 70°C / 40Pa, and collect this fraction. This liquid contains a small amount of water generated due to the reaction, and the resulting product is dissolved in THF, dried over anhydrous sodium sulfate, filtered with suction, and the solvent is removed by rotary evaporation to obtain a colorless liquid S-(+)-3-hydroxytetrahydrofuran (10g) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com