Preparation method of diastereoisomer 4-position substituted isoxazoline
A technology of diastereoisomers and isoxazolines, applied in the field of preparation of 4-position substituted isoxazolines, can solve the problem of simultaneously preparing diastereomer 4-position substituted isoxazolines and other issues to achieve the effect of broadening the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
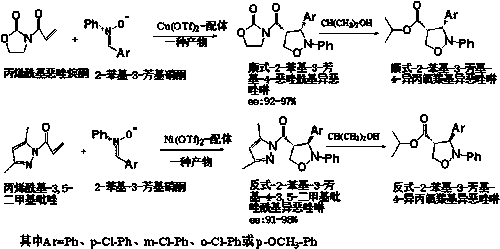
[0052] Specific embodiment one: the preparation method of diastereoisomer 4-position substituted isoxazoline in this embodiment is to use chiral bisoxazoline as catalyst ligand, respectively use Cu(OTf) 2 or Ni(OTf) 2 As a chiral metal catalyst, it catalyzes the reaction of acryloyl oxazolidinone and acryloyl-3,5-dimethylpyrazole with diaryl nitrone to prepare diastereomer 4-substituted isoxazolines, That is, the 4-position substituted isoxazoline in the cis configuration and the 4-position substituted isoxazoline in the trans configuration.
[0053] Among them, chiral bisoxazoline is used as catalyst ligand, and Cu(OTf) 2 As a chiral metal catalyst, it catalyzes the reaction of acryloyl oxazolidinone and diaryl nitrone to obtain a 4-position substituted isoxazoline with 100% cis configuration;
[0054] Using chiral bisoxazoline as catalyst ligand, Ni(OTf) 2 As a chiral metal catalyst, it catalyzes the reaction of acryloyl-3,5-dimethylpyrazole and diphenylnitrone to obtain ...
specific Embodiment approach 2
[0055] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the preparation method of the 4-position substituted isoxazoline in the cis configuration specifically includes the following steps:
[0056] Step 1: Under the protection of nitrogen, add the chiral metal catalyst and 4ÅMS into the round bottom flask, and then add CH 2 Cl 2 Inject the reaction system, replace the nitrogen three times, and then take the chiral catalyst ligand with CH 2 Cl 2 After dissolving, inject into the reaction system, stir at room temperature for 30~40min; wherein the chiral metal catalyst is Cu(OTf) 2 , the chiral catalyst ligand is chiral bisoxazoline;
[0057] Step 2: Take acryloyl oxazolidinone and use CH 2 Cl 2 After dissolving, inject into the system, stir at room temperature for 1~1.5h,
[0058] Adjust the reaction temperature to 0°C, take a certain amount of dipolar nitrone, and use CH 2 Cl 2 After dissolving, add dropwise to the reaction...
specific Embodiment approach 3
[0061] Embodiment 3: This embodiment is different from Embodiment 2 in that the molar ratio of the chiral metal catalyst to the chiral catalyst ligand in step 1 is 1:1. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com