A β-glucosidase with increased resistance to trypsin
A technology of glucosidase and trypsin, applied in the direction of enzyme, hydrolase, glycosylase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Synthesis of β-glucosidase gene
[0028] The present invention adopts the wild-type β-glucosidase gene (GenBank registration number: FJ882071.1) derived from Trichoderma viride, which is synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd. (other commercial companies with full gene synthesis can also complete it).
Embodiment 2
[0029] Example 2: Connection of β-glucosidase (bgl1) to cloning vector Taox+PgHT+PB
[0030] 1. The bgl1 target gene and the cloning vector Tao x +PgHT+PB were digested with the restriction enzymes EcoRI and SpeI / XbaI at 37°C for 10 min respectively. The digestion conditions were as follows:
[0031]
[0032] 2. After 1% agarose gel electrophoresis, the digested product was recovered and the two target fragments were respectively recovered and connected with T4 DNA ligase. The ligation system was as follows:
[0033]
[0034]
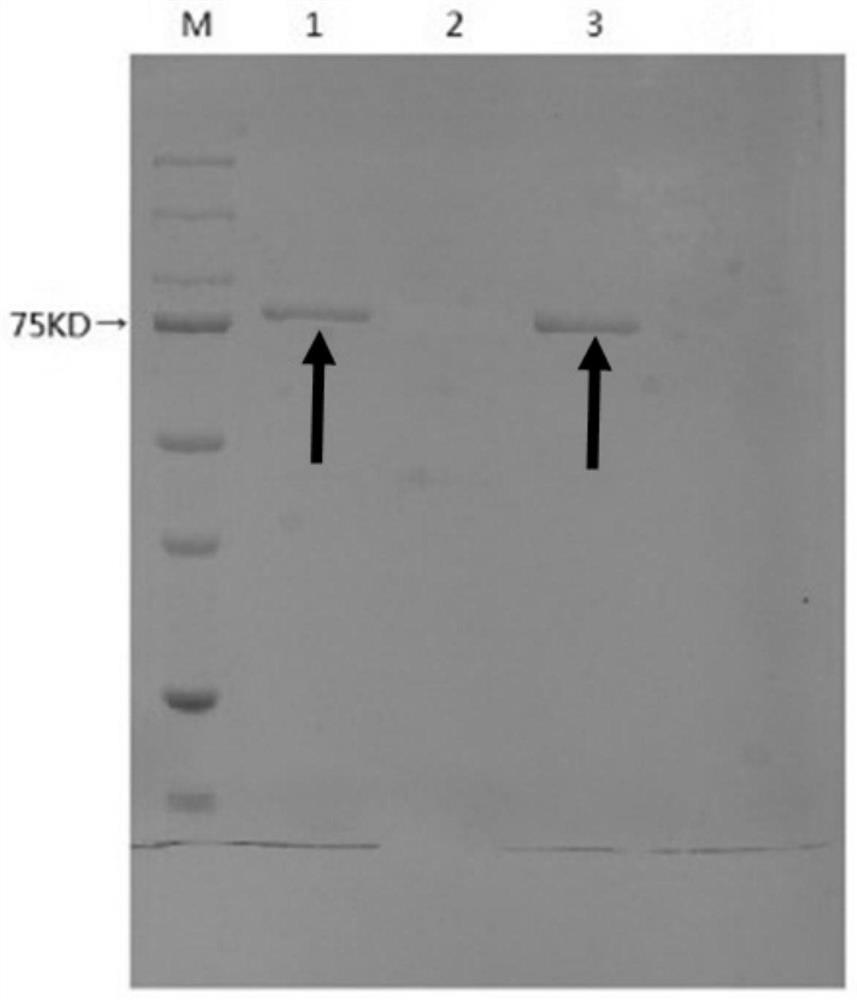
[0035] Ligase with ligase at 16°C for 12h, the ligation product was transformed into DH5a competent cells and amplified, and the plasmid was extracted with a plasmid extraction kit. After double digestion with EcoRI and PstI, the electrophoresis results showed that there were two bands of 7.0kb and 3.8kb. It indicated that the connection was successful, and it was determined to be a β-glucosidase gene by DNA sequencing.
Embodiment 3
[0036] Example 3: Gene fragment Paox+SS1 is connected to cloning vector M+Taox+PgHT+PB
[0037] 1. The gene fragment Paox+SS1 was transferred from the cloning vector Paox+SS1+PB preserved in this study, and was obtained by double digestion with EcoRI and SpeI endonucleases, purification and recovery;
[0038] 2. The cloning vector M+Taox+PgHT+PB was obtained in Example 2, and the connection method of the gene fragment Paox+SS1 and the cloning vector M+Taox+PgHT+PB was the same as that in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com