Phenylpropiolic acid small molecular organic compounds and synthetic method and application thereof
An organic compound, phenylpropiolic acid technology, applied in organic chemistry, organic active ingredients, medical preparations containing active ingredients, etc., can solve problems such as drug listing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0070] Embodiment 1-1, the preparation of compound 3-(4-(3-phenoxybenzyloxy)phenyl)propiolic acid (HL-001)
[0071]
[0072] Add 3-phenoxybenzaldehyde (1.20 g, 6.1 mmol) into a 50 mL round bottom flask, add 6 mL THF and 6 mL DME to dissolve it, and add NaBH in batches under ice-bath conditions 4 (464mg, 12.2mmol), continue to stir the reaction under ice bath for 3 hours. TLC detects and tracks the reaction. After the reaction is completed, slowly add an appropriate amount of deionized water to the reaction solution to quench, extract with ethyl acetate and water, take the organic phase and evaporate it to dryness, and purify it by column chromatography to obtain a colorless oil, namely 3-Phenoxybenzyl alcohol (1.20 g, 99.2% yield). Take the obtained 3-phenoxybenzyl alcohol (450mg, 2.3mmol) into a 50mL round-bottomed flask, dissolve it with 10mL of DCM, and add PBr dropwise under ice-bath conditions 3 liquid (0.3 mL, 2.5 mmol) and kept on ice for 3 hours. After the reaction...
Embodiment 2
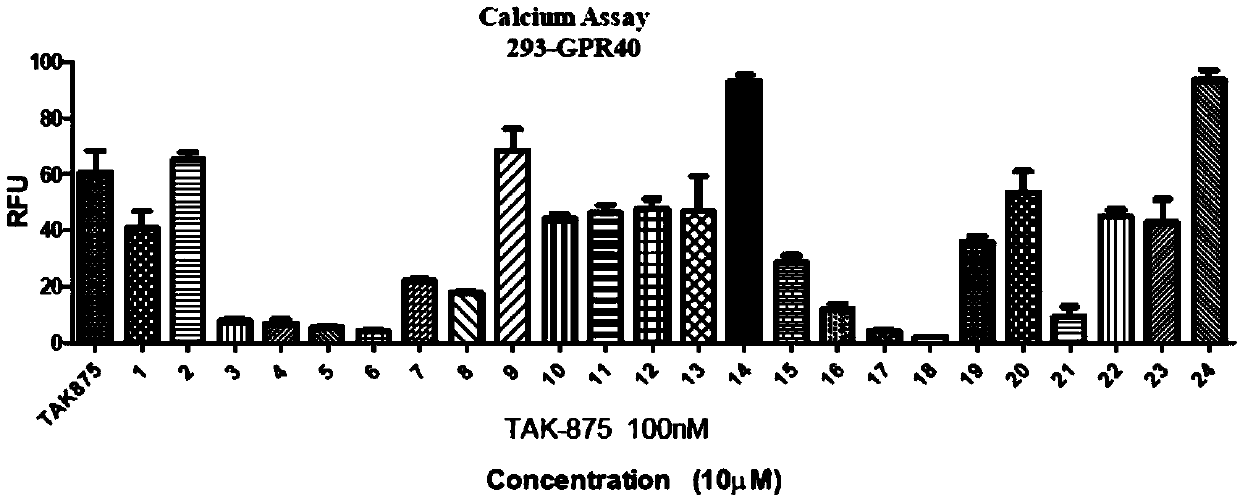
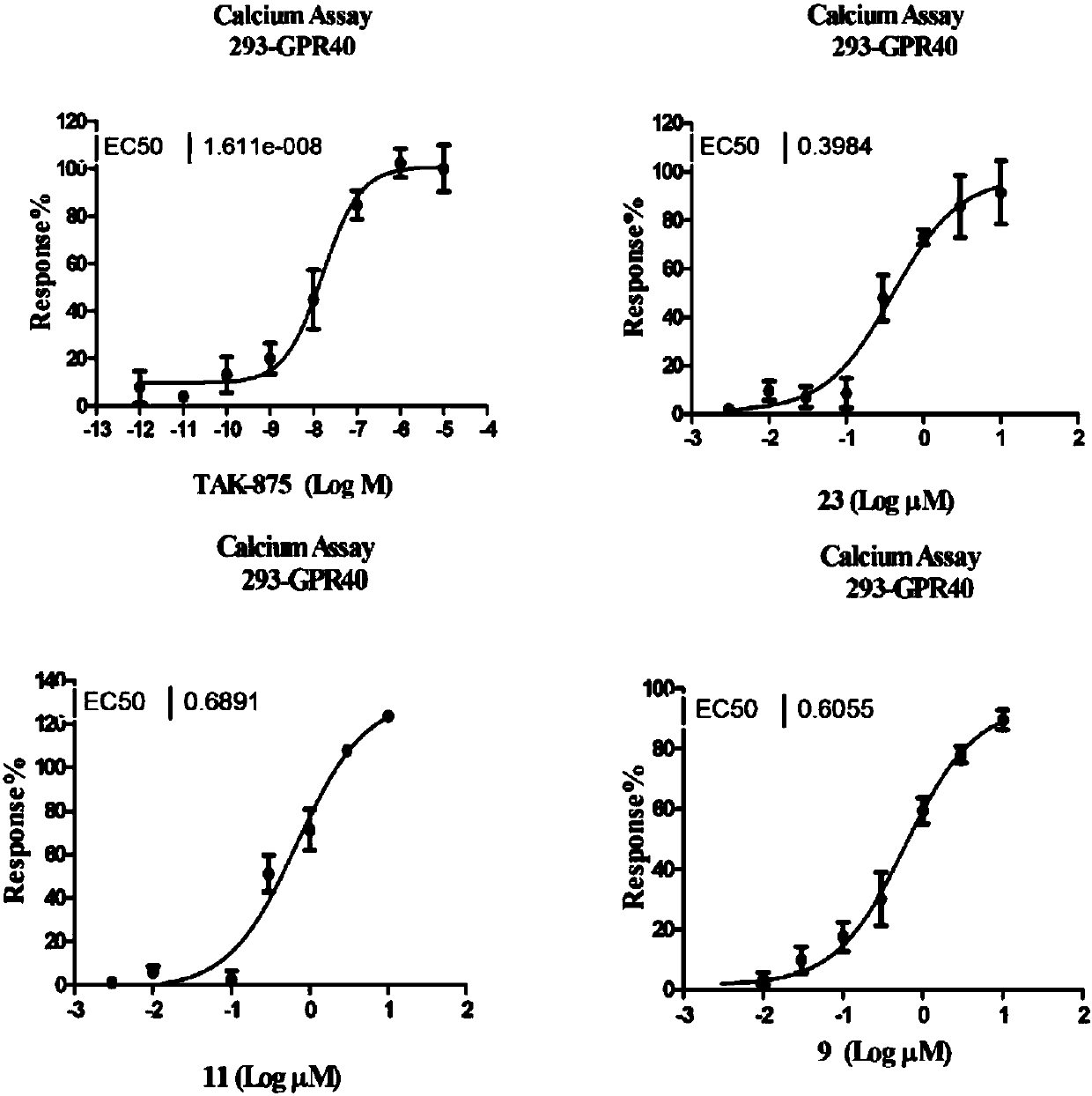
[0078] Example 2, the compound of the present invention has agonism to GPR40, resulting in intracellular Ca 2+ Concentration rises.
[0079] GPR40 coupled with Gαq can stimulate the release of endoplasmic reticulum calcium flow after being activated. It is a widely used GPR40 ligand screening method to reflect the activation state of GPR40 by detecting intracellular calcium flow. The principle of the calcium flow experiment is to introduce a fluorescent dye sensitive to calcium ions into the cells to detect the changes of calcium ions in the cells. Calcium flux assays are based on the detection of second messengers. When screening agonists, the test compound is added to the system. If the compound has an agonistic effect on the receptor, it will cause intracellular Ca 2+ When the concentration increases and the fluorescence intensity increases, the agonistic activity of the test compound on GPR40 can be calculated by calculating the increase of the fluorescence intensity.
...
Embodiment 3
[0091] Example 3. Target verification and in vitro functional experiments of phenylpropiolic acid small molecule organic compounds of the present invention
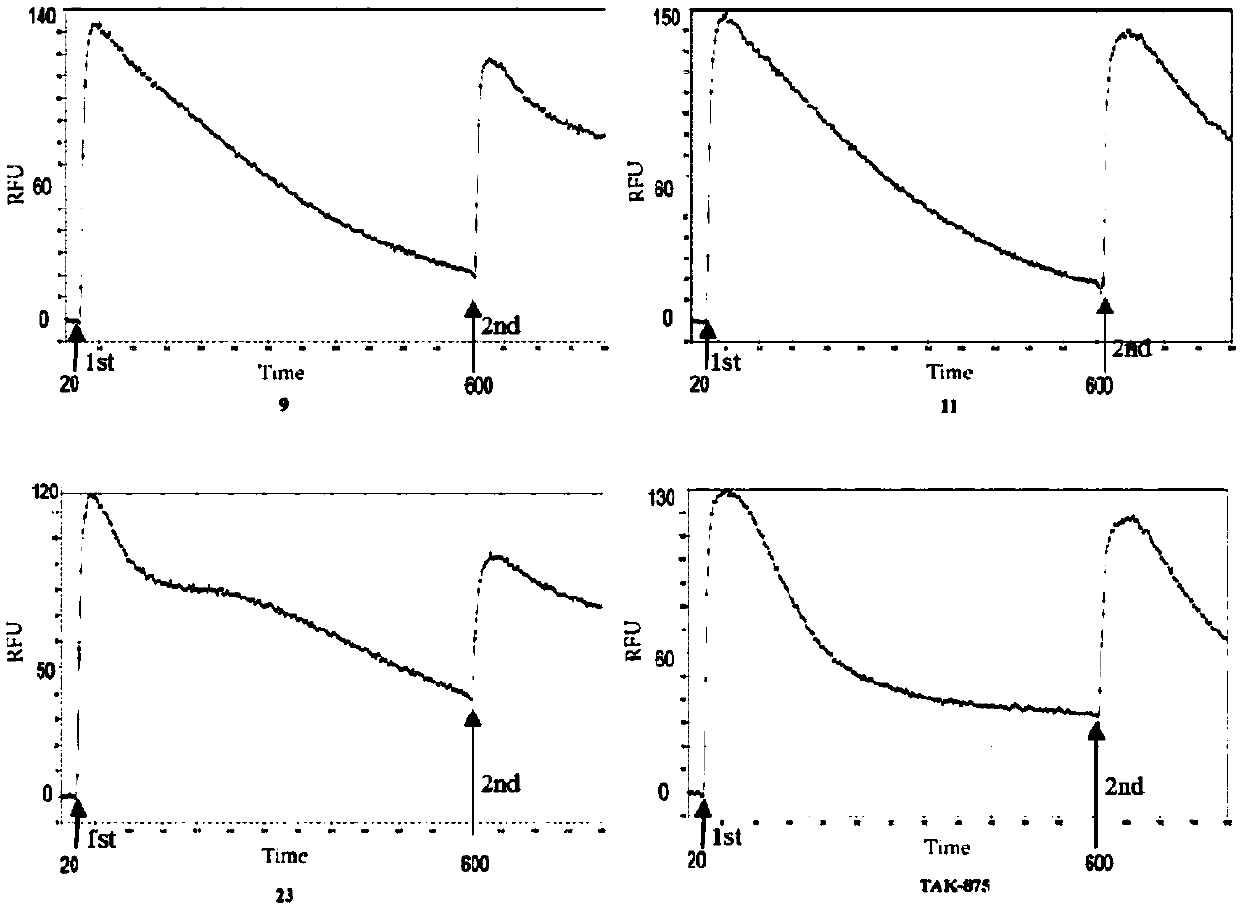
[0092] After obtaining the activity screening results of the phenylpropiolic acid small molecule organic compound of the present invention, the present invention continues to confirm the target. The loss of GPCR function after agonist stimulation is a common mechanism for receptor desensitization and internalization. Since a series of compounds prepared by the present invention have similar structures and belong to one type of compound, the inventors selected one of them with relatively high activity. The good three compounds HL-009, HL-011, HL-023 verified whether they can induce GPR40 receptor desensitization and internalization.
[0093] In the present invention, TAK-875 (1 μM), HL-009 (10 μM), HL-011 (10 μM) and HL-023 (10 μM) are used for the first stimulation respectively. Such as image 3 As shown, it can be foun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com