Rapamycin cataplasms and preparation method thereof
A technology of rapamycin and cataplasm, which is applied in the field of rapamycin cataplasm and its preparation, can solve problems such as low immune function and increased risk of diabetes, and achieve improved targeting, increased risk of diabetes, and delay The effect of wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Preparation example of rapamycin micelles:
[0031] ①Stir 0.1g of rapamycin, 1.5g of Tween-80 and 1g of ethanol at room temperature for 5 minutes; slowly add 7ml of water during the stirring process, and the water addition time is 8 minutes, continue to stir for 20 minutes and let stand for 30 minutes, The rapamycin micelles 1 were obtained, and the average particle size of the rapamycin micelles 1 was about 19 nm.
[0032] ②Preparation of rapamycin micelles: Stir 0.2g of rapamycin, 2.5g of polyoxyethylene castor oil and 1g of n-propanol at room temperature for 4min; slowly add 5ml of water during stirring, add water The time is 10 minutes, continue to stir for 15 minutes and stand still for 30 minutes to obtain rapamycin micelles 2, and the average particle size of rapamycin micelles 2 is about 23 nm.
Embodiment 2
[0033] Embodiment 2: Preparation example of rapamycin cataplasm:
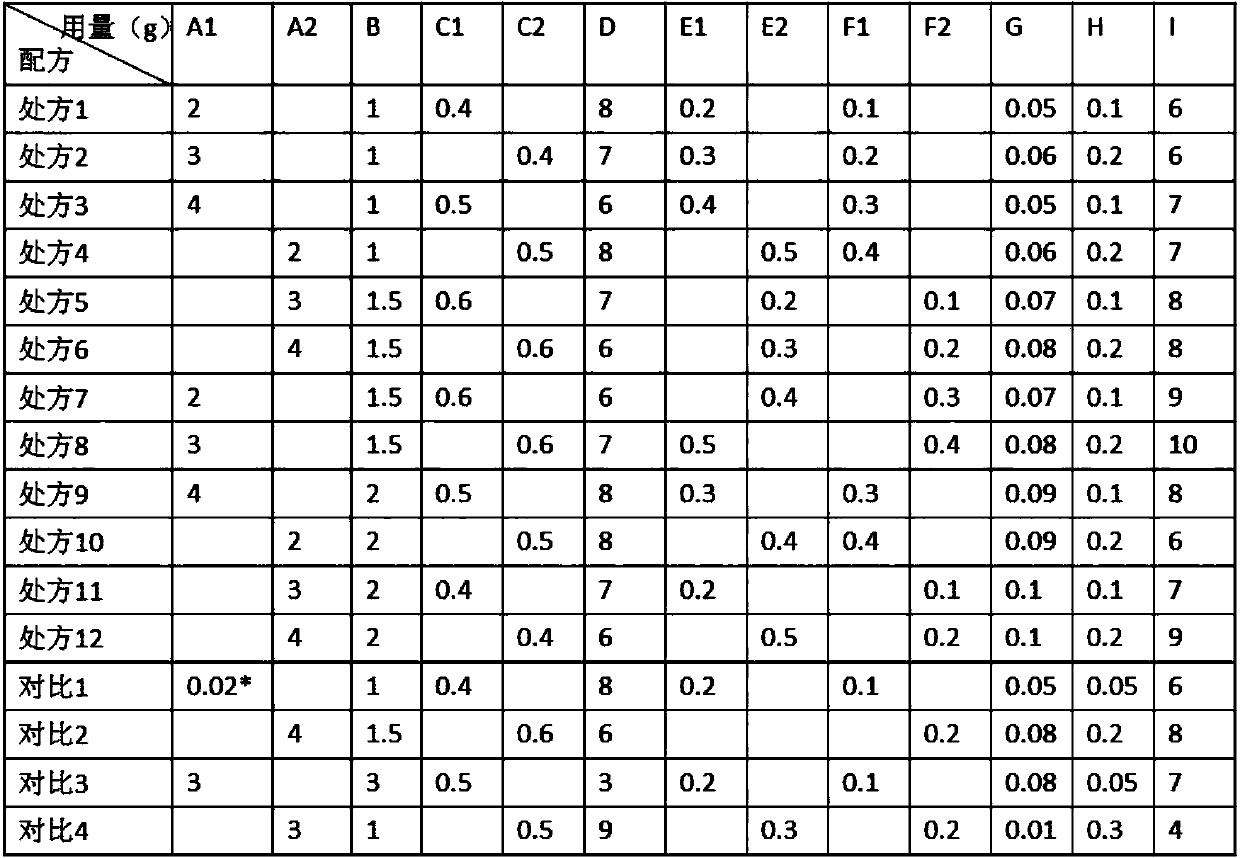
[0034] Rapamycin micelles 1 and rapamycin micelles 2 are denoted as A1 and A2 respectively; the hydrophilic polymer is sodium polyacrylate NP700 and denoted as B; the thickener sodium carboxymethyl cellulose and PVP are respectively Denote as C1 and C2; moisturizer glycerin as D; oil phase matrix oleic acid and linoleic acid as E1 and E2 respectively; transdermal penetration enhancer as Azone and menthol as F1 and F2 respectively; It is G; pH adjuster tartaric acid is denoted as H; pure water is denoted as I.
[0035] The composition of rapamycin cataplasm is as shown in table 1:
[0036] Table 1: Composition of rapamycin cataplasm
[0037]
[0038] Note: the A1 component of comparison 1 is the direct addition of 0.02g rapamycin
[0039] The preparation method of rapamycin cataplasm: mix the hydrophilic polymer and the thickener evenly and add water to dissolve to form phase I; mix the humectant and alumi...
Embodiment 3
[0041] Example 3: Adhesion evaluation and pH of rapamycin cataplasm
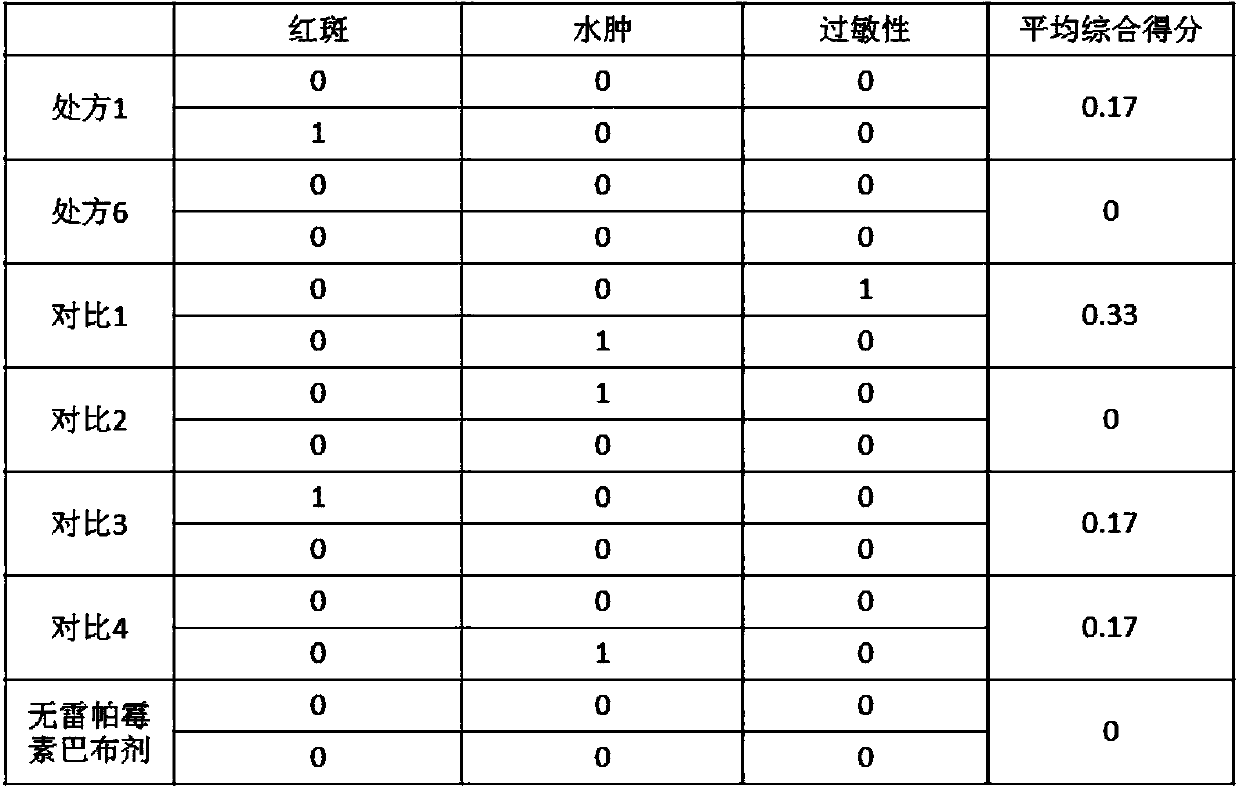
[0042]According to the "Chinese Pharmacopoeia" 2010 edition one appendix ⅫE adhesion determination method, measure the initial adhesion and holding force of rapamycin cataplasm; according to the "Chinese Pharmacopoeia" 2010 edition two appendix VIH pH value Assay operation, the pH value of measuring rapamycin cataplasm, the results are as shown in table 2:
[0043] Table 2: Adhesion test of rapamycin cataplasm
[0044]
[0045] It can be seen from the data in the table that the difference between the cataplasms of comparison 1 and 2 lies in whether they contain micelles and oil phase matrix, so the adhesion and pH value of the cataplasms are similar to those of the corresponding formulations 1 and 6. Contrasts 3 and 4 have different amounts of components, and the obtained cataplasms have poor adhesion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com