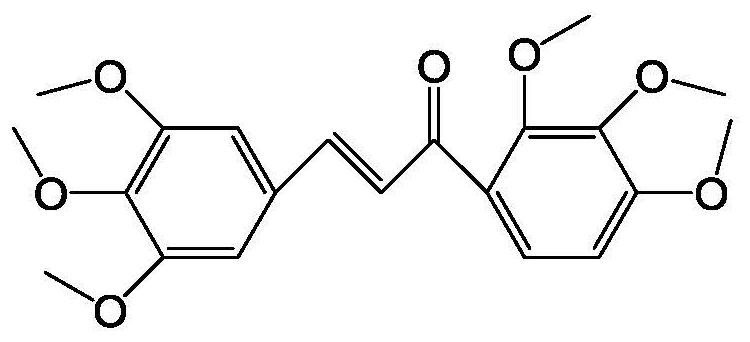
Application of (1,2,3-trimethoxybenzene)-propenone in the preparation of drugs for improving neurotoxicity caused by glutamic acid
A technology of trimethoxybenzene and neurotoxicity, applied in the fields of biology and medicine, can solve the problems of mitochondrial function damage, mitochondrial membrane permeabilization, cell death, etc., achieve significant neuroprotective function, inhibit neurotoxicity, and have good application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] (1,2,3-Trimethoxybenzene)-propenone has no toxic and side effects on mouse hippocampal neurons and human neuroblastoma cells
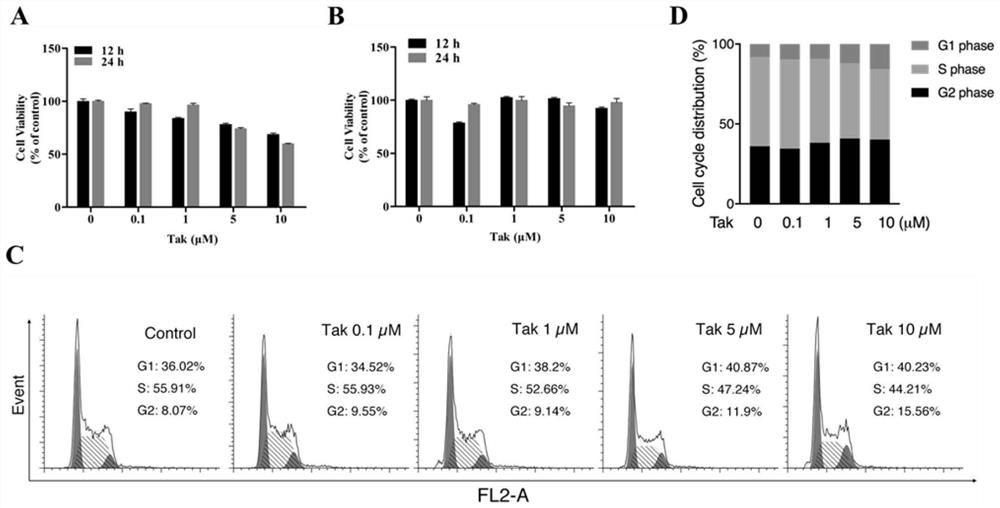
[0084] Use (1,2,3-trimethoxybenzene)-propenone to act on mouse hippocampal neuron cells and human neuroblastoma cells for 12h or 24h, and detect cell viability. figure 2 A and figure 2 B shows that (1,2,3-trimethoxybenzene)-propenone at concentrations below 5 μM has no effect on cell viability of mouse hippocampal neurons, and (1,2,3-trimethoxybenzene)-propenone at concentrations above 5 μM Acrylone can slightly reduce the viability of mouse hippocampal neurons, but (1,2,3-trimethoxybenzene)-propenone does not affect the viability of human neuroblastoma cells. results from the cell cycle figure 2 C and figure 2 D It can be seen that high concentration of (1,2,3-trimethoxybenzene)-propenone can arrest the cell cycle of mouse hippocampal neurons in the S phase, thereby inhibiting cell proliferation and reducing cell viability.
Embodiment 2
[0086] (1,2,3-Trimethoxybenzene)-propenone improves mitochondrial function
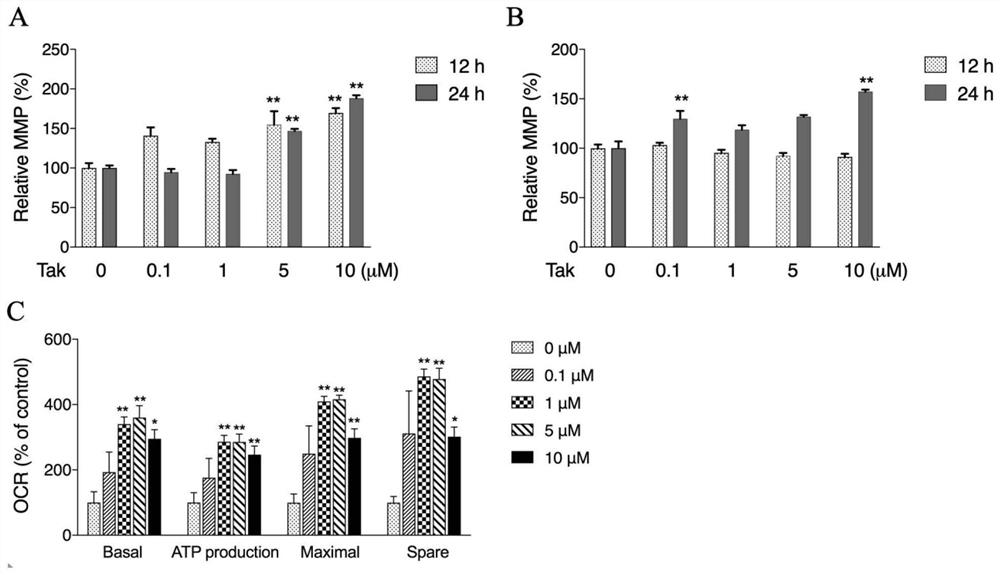
[0087] Use (1,2,3-trimethoxybenzene)-propenone to act on mouse hippocampal neuron cells and human neuroblastoma cells for 12h or 24h, and detect the mitochondrial membrane potential. image 3 A and 3B show that (1,2,3-trimethoxybenzene)-propenone can significantly increase the mitochondrial membrane potential of mouse hippocampal neuron cells and human neuroblastoma cells. image 3 C is the result graph of mitochondrial respiration oxygen consumption rate in mouse hippocampal neuron cells treated with (1,2,3-trimethoxybenzene)-propenone, and the mitochondrial respiration oxygen consumption rate represents the ability of mitochondria to carry out aerobic metabolism. Such as image 3 C shows the basal oxygen consumption, oxidative phosphorylation oxygen consumption for ATP synthesis, maximal respiratory oxygen consumption and non-mitochondrial oxygen consumption in the (1,2,3-trimethoxybenzene)-propeno...
Embodiment 3
[0089] (1,2,3-Trimethoxybenzene)-propenone is an antioxidant targeting mitochondria
[0090] Intracellular reactive oxygen species are mainly produced by the mitochondrial respiratory chain as well as NADPH oxidase and peroxidase. As the main site of reactive oxygen species generation, mitochondria with high aerobic capacity will generate more reactive oxygen species. Therefore, the ability of (1,2,3-trimethoxybenzene)-propenone to scavenge reactive oxygen species was detected by using the reactive oxygen species probe DCFH-DA and the mitochondrial reactive oxygen species probe MitoSOX. Figure 4 A shows that compared with the control, 5 μM (1,2,3-trimethoxybenzene)-propenone can significantly reduce the level of reactive oxygen species in cells, suggesting that it has antioxidant effect. Simultaneously, Figure 4 B shows that compared with the control, the weakening of the red fluorescence intensity proves that (1,2,3-trimethoxybenzene)-propenone can obviously remove the mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com