Application of compound in antagonization of accumulation of beta amyloid proteins
An amyloid protein and compound technology, which is applied in the fields of drug combination, ketone active ingredients, nervous system diseases, etc., can solve the problems that have not been reported, and achieve the obvious effect of fiber structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
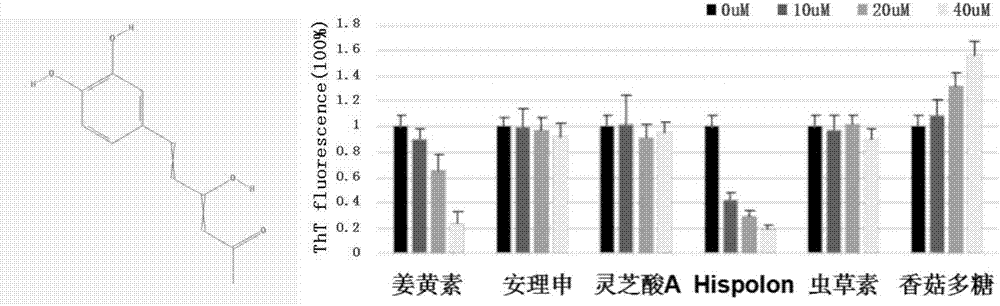
[0023] Thiothioflavin T experiment of embodiment 1 Hispolon and Aβ protein
[0024] The protein Aβ protein was synthesized by Nanjing Taopu Biological Co., Ltd., with a purity greater than 95%. The compound Hispolon was provided by ENZO Biological Company of the United States. The Aβ monomer powder is stored at -80°C. When it is taken out for the first time, it should be allowed to stand at room temperature for 30 minutes, then diluted with hexafluoroisopropanol (HFIP) to 1.0 mg / ml, left to stand for at least 2 hours, and ultrasonicated for 30 minutes to destroy the existing excess Polymer structure, after drying at low temperature, store at -20°C immediately. Thioflavin T (Thioflavin T) was purchased from Sigma (product number T3516-5g). Weigh 0.032g of ThT powder and dissolve it in 20ml of 20mM Tris-HCl (pH 7.4) solution to obtain 5mM ThT mother solution, which is diluted to 5uM when used.
[0025] When used, the HFIP-treated Aβ protein and the compound Hispolon were diss...
Embodiment 2
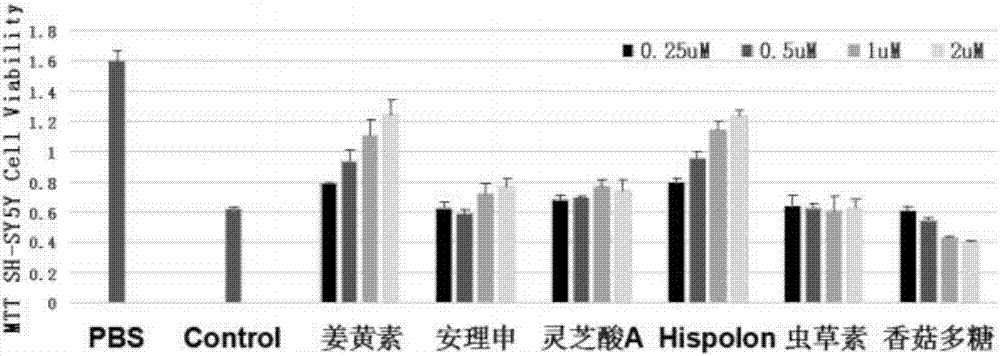
[0027] Example 2 Hispolon and Aβ protein-mediated SH-SY5Y cytotoxicity experiment
[0028] The cells used in the experiment were human neuroma blasts SH-SY5Y, cultured in DMEM medium with 10% FBS at 37°C. The Aβ protein control group without drugs and the drug-added experimental group mother solution were cultured overnight at 37°C with slow shaking, and transferred to a 96-well cell culture plate containing SH-SY5Y. The final concentration of Aβ protein was 1uM, and the final concentration of drug was 0.25uM. 0.5uM, 1uM and 2uM, three parallel wells were set for each concentration, the action time was 3 hours, and the MTT method was tested 5 times at 490nm wavelength to analyze the cell proliferation.
[0029] Using the above method, the results of the absorbance values of each group detected are shown in the attached figure 2 . It can be seen that compared with the control group without Hispolon, the absorbance value of the experimental group added with Hispolon compoun...
Embodiment 3
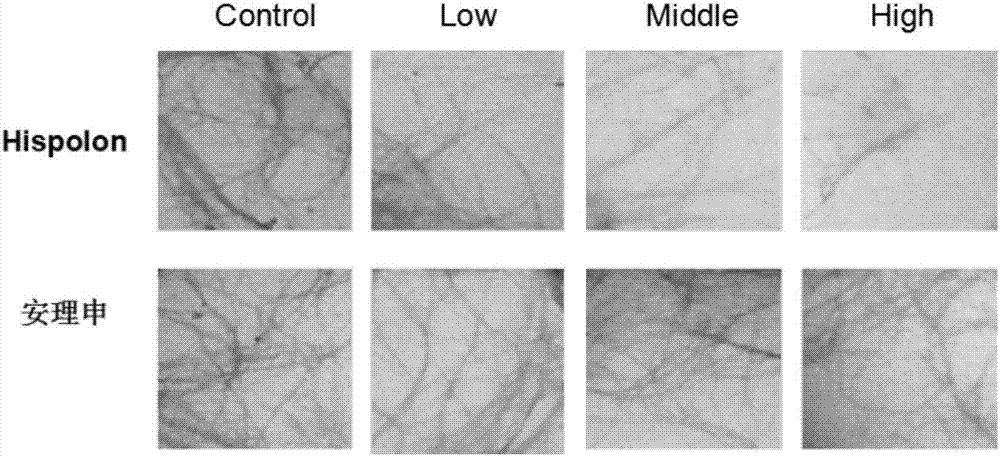
[0030] Example 3 Hispolon and Aβ Protein Transmission Electron Microscopy Experiment (Transmission electron microscopy, TEM)
[0031] As in Example 1, 5ul of the Aβ protein sample incubated at 37°C for 24 hours was dropped on the surface of a 300-mesh carbon film copper grid, air-dried, negatively stained with 2% (w / v) sodium phosphotungstic acid for 30 seconds, and left at room temperature. After standing still for about 4 hours, the JEM-100CXII transmission electron microscope system (JEOL inc., Tokyo, Japan) was used to detect and take pictures, and the accelerating voltage was 80kV.
[0032] The observation results are attached image 3 . Compared with the untreated control group, the density, length and width of Aβ protein formed fibers in the experimental group adding Hispolon compounds were significantly decreased in a concentration-dependent manner, while the other group of cholinesterase inhibitors Aricept (donepezil hydrochloride) Inhibition of fibril formation by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com