Application of matrine in preparing drug for preventing and treating alzheimer disease
A technology of Alzheimer's and matrine, applied in the field of drug research, can solve the problems such as difficulty in obtaining recognition of traditional Chinese medicine for AD treatment, few theoretical and experimental studies, lack of scientificity and persuasion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: Effect Analysis of Matrine Inhibiting or Neutralizing the Cytotoxicity of Aβ42 Monomers and Aβ42 Aggregates
[0015] (1) Preparation of Aβ42 monomer and Aβ42 oligomer
[0016] Aβ42 (purchased from Sigma-Aldrich, USA) was dissolved in ice-precooled hexafluoroisopropanol (HFIP) to a concentration of 1 mg / mL, sonicated in an ice-water bath for 10 min, dried in vacuum, and frozen at -20 °C. When using, first dissolve Aβ42 with dimethyl sulfoxide (DMSO) to a concentration of 1 mg / ml, and then dilute Aβ42 to a concentration of 10 μM in phosphate buffer (pH 7.4, 50 mM), with a final concentration of 20 μM. When the Aβ42 was regarded as Aβ42 monomer; the prepared Aβ42 was incubated at 37°C for 12h to form the aggregation state of Aβ42 oligomers, and the aggregation state was confirmed by transmission electron microscopy ( figure 1 ). Due to the irreversibility of Aβ42 to form aggregates, the use of Aβ42 oligomers is ready-to-use and can be stored at -80°C for short...
Embodiment 2
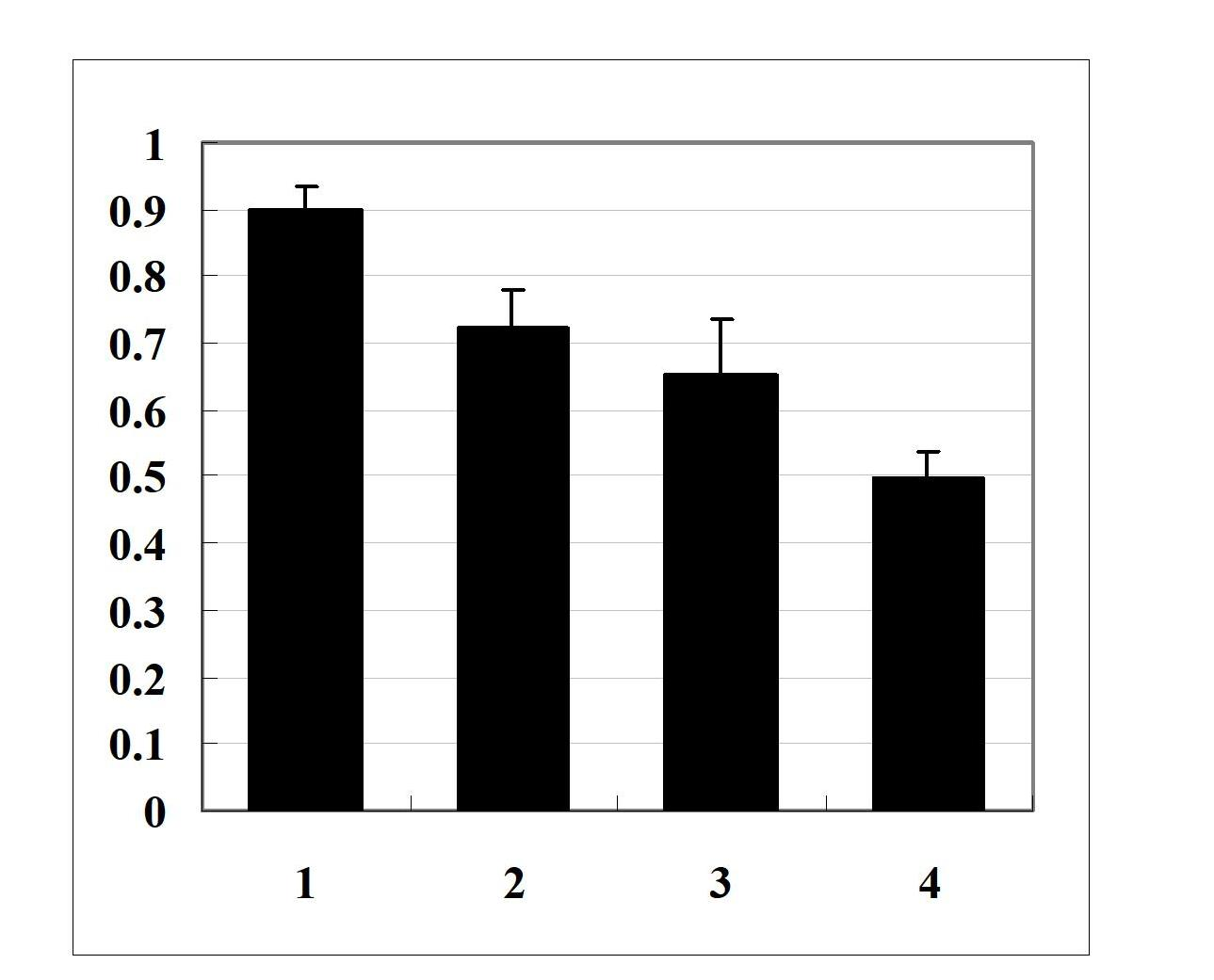
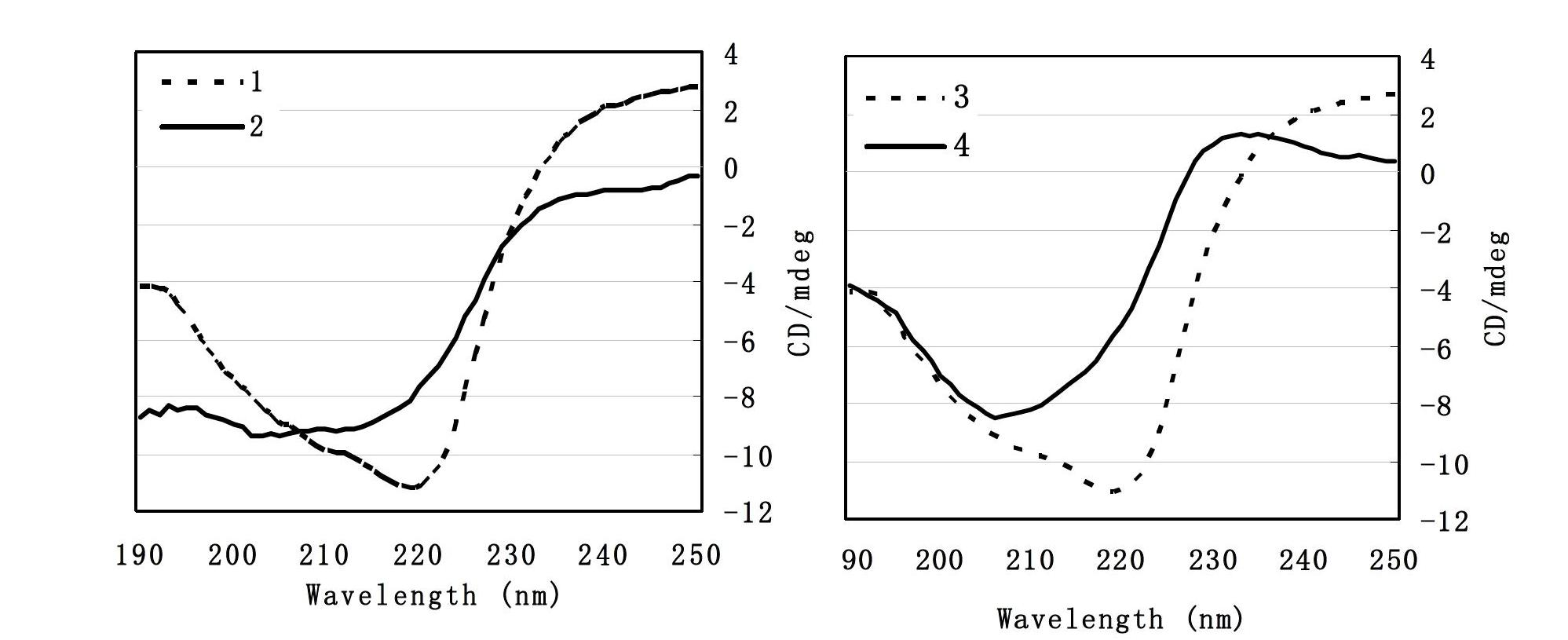
[0022] Example 2: Effects of Matrine on the Secondary Structure of Aβ42 Monomers and Aβ42 Oligomers
[0023] For the preparation method of Aβ42 monomer and Aβ42 oligomer, see Example 1 (1). 40 μM Aβ42 monomer or Aβ42 oligomer was mixed with 500 uM matrine, incubated at 37°C for 24 hours, and incubated at the same concentration and Time Aβ42 monomer or Aβ42 oligomer solution was used as control. The experiment was divided into four groups, namely 1: Aβ42 oligomer group, 2: (Aβ42 oligomer + matrine) group, 3: Aβ42 monomer group, 4: (Aβ42 monomer + matrine) group ; Inject the sample into a 0.1cm thick oval cuvette, and scan in the range of 190-260nm at 25°C under the condition of continuous nitrogen filling. JASCOJ-500 polarimeter was used to measure the changes of far-ultraviolet circular dichroism spectrum of Aβ42 under different aggregation states.
[0024] Molecular ellipticity [θ] calculation formula: [θ]=θ / (10CL); θ: ellipticity unit (mdeg) measured by JASCOJ-500 polarime...
Embodiment 3
[0026] Example 3: Transmission Electron Microscopy Observation of the Effect of Matrine on the Aggregation Form of Aβ42 Monomers and Aβ42 Oligomers
[0027] For the preparation method of Aβ42 monomer and Aβ42 oligomer, see Example 1 (1). Aβ42 monomer is prepared into a solution with a concentration of 20 μM in phosphate buffer (50 mM, pH 7.4), and mixed with 200 μM matrine Incubate at 37°C for 24h; in the same way, mix the prepared Aβ42 oligomer with a concentration of 20μM and 200μM matrine and incubate at 37°C for 12h. treatment as a control. The experiment was divided into four groups, namely 1: Aβ42 oligomer group, 2: (Aβ42 oligomer + matrine) group, 3: Aβ42 monomer group, 4: (Aβ42 monomer + matrine) group ;After the sample is processed, drop it on the carbon film of the copper grid, blot the excess liquid with filter paper, dry it in the air for 1min, and negatively stain it with 2% (w / v) phosphotungstic acid, TEM-1200EXⅡ type transmission electron The morphology of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com