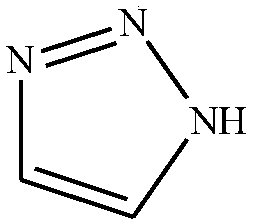
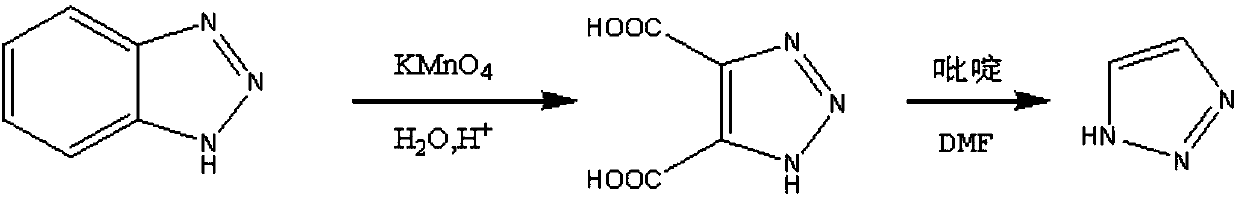
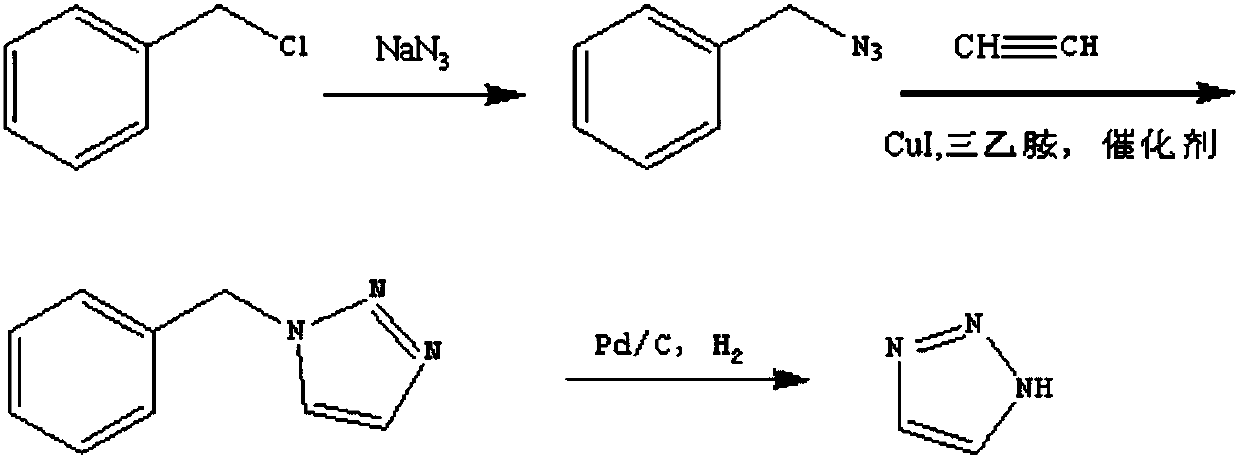
Method for preparing 1H-1,2,3-triazole
A 1H-1, triazole technology, applied in the field of medicine and chemical industry, can solve the problems of high price, difficult to buy starting materials, unsuitable for industrialized large-scale production, etc., achieves simple operation, safe and environmentally friendly synthesis process, broad market prospects and economic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0048] In a 500ml three-necked flask, add 150ml water, 107.5g (1.72mol) 80% hydrazine hydrate solution, stir, and cool to -5~5°C. 100g (0.69mol) of 40% glyoxal solution was added dropwise, and a white solid gradually precipitated in the feed solution. After dripping, react at room temperature for 2h. Crystallize at a temperature below 0°C for 3h, filter, rinse the filter cake with 30ml of cold methanol to obtain a white wet product, and vacuum dry at 30-40°C for 5h. 54.2 g of white crystalline solid was obtained, with a yield of 91.3%, which is the intermediate one.
[0049] In a 1000ml three-necked flask, add 300ml of dichloromethane, 50.0g (0.58mol) of Intermediate One, control the temperature at 25~35℃, stir until the material liquid is clear, add 110g (0.70mol) potassium permanganate in 5 times to react The liquid gradually turned dark red. After the addition, the reaction was kept for 3 hours. After the reaction was completed, a light red filtrate was obtained by filtratio...
Embodiment example 2
[0053] In a 2000ml three-necked flask, add 750ml of water and 538.0g (8.61mol) of 80% hydrazine hydrate solution, stir and cool to -5~5°C. 500.0g (3.45mol) of 40% glyoxal solution was added dropwise, and a white solid gradually precipitated in the feed liquid. After dripping, react at room temperature for 2h. Crystallize at a temperature below 0°C for 3h, filter, and rinse the filter cake with 150ml of cold methanol to obtain a white wet product, which is dried under vacuum at 30-40°C for 5h. 273.2 g of a white crystalline solid was obtained with a yield of 92.1%, which is the intermediate one.
[0054] In a 3000ml three-necked flask, add 1620ml of dichloromethane, 270.0g (3.13mol) of Intermediate One, control the temperature at 25~35℃, stir until the material liquid is clear, add 595.0g (3.76mol) potassium permanganate in 5 times, The reaction solution gradually turned dark red. After the addition, the reaction was kept for 3 hours. After the reaction was completed, a light re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com