Synthesis and application of a class of antibacterial drug asc for targeted treatment of methicillin-resistant staphylococcus aureus, staphylococcus aureus and superbug infection
一种药物、反应的技术,应用在抗细菌药、含有效成分的医用配制品、医药配方等方向,能够解决无效等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
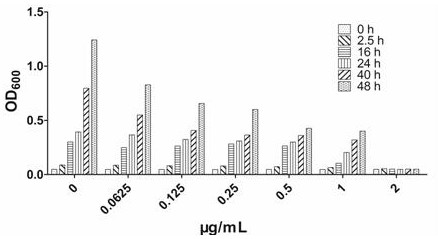
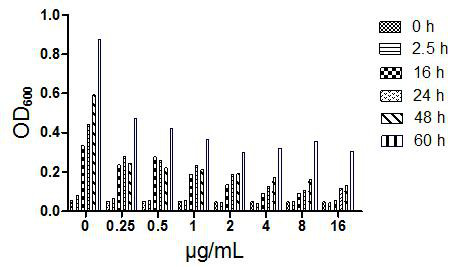
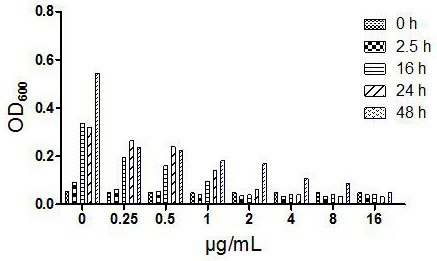
Image
Examples
Embodiment 1
[0052] The structural formula of ASC-NB of the present invention is as follows:
[0053]
[0054] The above-mentioned ASC-NB is prepared according to the following steps:
[0055] 1. The preparation steps of intermediate product C4 are as follows:
[0056]
[0057] (1) Mix C1 and hydrazine hydrate at a molar ratio of 1:1, heat up to 80°C and reflux for 8-12h. After the reaction is completed, it is slowly cooled to room temperature, and a large amount of white solid C2 is generated during the cooling process;
[0058] 1 H NMR (400MHz, DMSO-d 6 ): δ12.52(s,1H), 10.07(s,1H), 7.79(d,1H), 7.37(t,1H), 6.87(dd,2H), 4.65(s,2H).
[0059] (2) Dissolve C2 in a sufficient amount of ethanol, stir at room temperature until clear, add ammonium thiocyanate in an equimolar ratio, then add concentrated hydrochloric acid and heat up to 80-100°C for reflux for 12-16h. After the reaction was completed, it was cooled to room temperature, the insoluble matter was removed by filtration, an...
Embodiment 2
[0073] The structural formula of ASC-NA of the present invention is as follows:
[0074]
[0075] 1. the preparation step of intermediate product D3,
[0076]
[0077] (1) Mix D1 and thiosemicarbazide in acetonitrile at a molar ratio of 1:1 and reflux for 2 hours. After cooling, the obtained solid is washed repeatedly with ethanol to obtain the intermediate product D2.
[0078] (2) Dissolving D2 in sodium hydroxide solution, refluxing overnight, adjusting the pH to acidic with dilute hydrochloric acid to obtain D3;
[0079] 1 H NMR (400MHz, CDCl 3 ): δ 3.26 (s, 1H), 2.53 (t, J = 5.9Hz, 2H), 2.31 (t, J = 5.5Hz, 2H), 1.89 (p, J = 5.7Hz, 2H).
[0080] 2. the preparation step of intermediate product D4,
[0081]
[0082] (1) Dissolve A1 and 2,6-lutidine in acetonitrile, add 1.5 equivalents of acid chloride dropwise at 0°C for 2 hours, then add an equivalent amount of triethylamine, stir overnight and then warm to room temperature. TLC tracking, after the reaction was c...
Embodiment 3
[0091] ASC-SB structural formula of the present invention is as follows:
[0092]
[0093] 1. the preparation step of intermediate product E4,
[0094]
[0095] (1) Mix E1 and hydrazine hydrate at a molar ratio of 1:1, heat up to 80-100°C and reflux for 8-10 hours. After the reaction was completed, it was cooled to room temperature, and a large amount of white solid E2 was obtained after cooling.
[0096] (2) Dissolve E2 in a sufficient amount of ethanol, stir at room temperature until clarified, then add an equivalent amount of ammonium thiocyanate, then add concentrated hydrochloric acid to raise the temperature to 80-100°C and reflux for 12-16h. After the reaction was completed, it was cooled to room temperature, the insoluble matter was removed by filtration, the filtrate was spin-dried under reduced pressure, and dried in vacuum to obtain E3.
[0097] (3) Dissolve E3 in a small amount of concentrated hydrochloric acid, stir and reflux. After the reaction was comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com