Kit for radionuclide labeled antibody and application thereof
A radionuclide and labeled antibody technology, applied in the direction of radioactive carriers, peptides, specific peptides, etc., can solve the problems of unstable yield, long time consumption, and high requirements for storage conditions, and achieve high in vitro stability and technological conditions. Stable, high-resolution images
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1) Preparation of labeled precursor
[0036] Take the NaHCO whose concentration is 0.1mol / L pH=8.4 3 solution to dilute the aqueous solution of antibody KN035 so that the concentration of KN035 is 10mg / mL, and then use 0.1mol / L Na 2 CO 3 Adjust the pH of the solution to 9.0; then add a DMSO solution of the chelating agent Df-Bz-NCS at a concentration of 2 mol / mL, so that the molar ratio of Df-Bz-NCS to KN035 is 5:1, after mixing, at 37 ° C After reacting for 60 minutes, the crude product of the labeled precursor Df-Bz-NCS-KN035 was obtained.
[0037] The obtained crude product was purified by a PD10 column eluting with sodium acetate, and was used after purification.
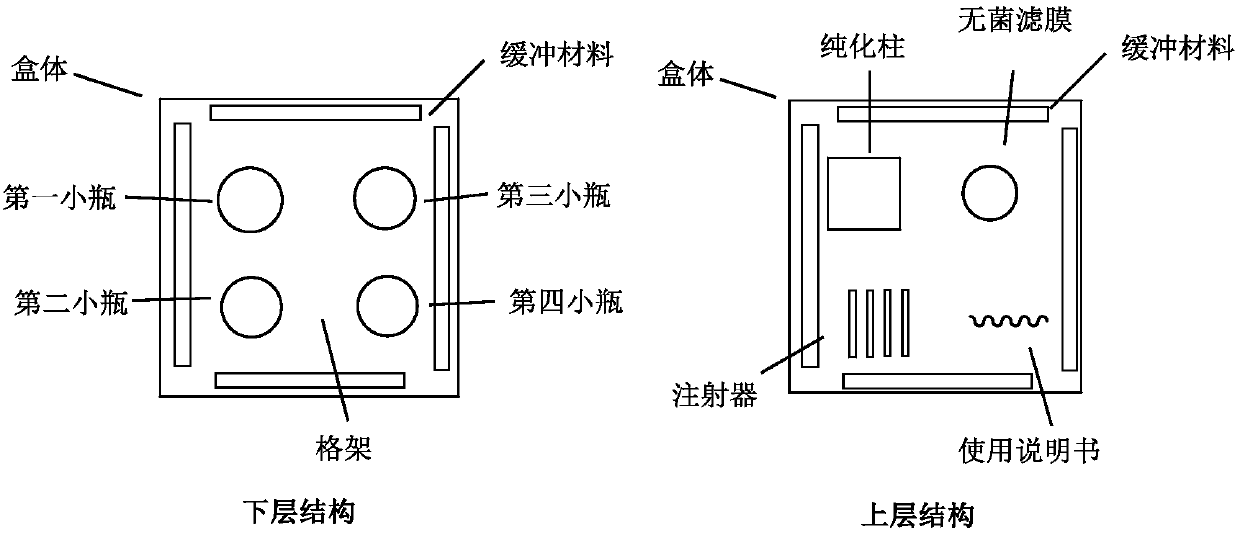
[0038] 2) Kit composition
[0039] a. The first vial: containing Df-Bz-NCS-KN035 3mg
[0040] b. The second vial: 5ml of acetic acid-sodium acetate buffer solution with pH=6.5
[0041] c. The third vial: containing 1ml of sodium carbonate solution
[0042] d. Fourth vial: 50ml of acetic acid-sodium...
Embodiment 2
[0062] 1) Preparation of labeled precursor
[0063] In phosphate buffered saline (PBS), the molar ratio of the chelating agent DOTAGA to the antibody Denosumab was 10:1, and reacted at 37°C for 2 hours to obtain the crude product of the labeled precursor Denosumab-DOTAGA.
[0064] The obtained crude product was purified by using a PD10 column eluted with physiological saline, and was used after purification.
[0065] 2) Composition of the kit
[0066] a. The first vial: containing Denosumab-DOTAGA 1mg
[0067] b. The second vial: 3ml of sodium acetate buffer containing pH=5.0
[0068] c. The third vial: containing 1ml of sodium bicarbonate solution
[0069] d. The fourth vial: containing 50ml of physiological saline buffer
[0070] It also includes 1 Hitrap Desalting desalting column, 1 sterile filter membrane, 4 syringes, and instructions for use.
[0071] 3) Use of the kit
[0072] a. Add 5mCi 64 The Cu solution was injected into the acetic acid-sodium acetate buffer ...
Embodiment 3
[0079] 1) Preparation of labeled precursor
[0080] Take the NaHCO whose concentration is 0.1mol / L pH=8.4 3 The solution dissolves the antibody Ipilimumab, so that the concentration of Ipilimumab is 10mg / mL, and then 0.1mol / L Na 2 CO 3 Adjust the pH of the solution to 9.0; then add a DMSO solution of the chelating agent Df-Bz-NCS at a concentration of 2mol / mL to it, so that the molar ratio of Df-Bz-NCS to Ipilimumab is 5:1, after mixing, at 37°C After reacting for 60 minutes, the crude product of the labeled precursor Df-Bz-NCS-Ipilimumab was obtained.
[0081] The obtained crude product was purified by a PD10 column eluting with sodium acetate, and was used after purification.
[0082] 2) Composition of the kit
[0083] a. The first vial: containing Df-Bz-NCS-Ipilimumab 2mg
[0084] b. The second vial: 5ml of sodium citrate buffer containing pH=7.0
[0085] c. The third vial: containing 1ml of sodium carbonate solution
[0086] d. The fourth vial: containing sodium cit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com