Substituted pyridazinone compound
A compound, solvent compound technology, applied in the field of medicine, can solve problems such as poor absorption, distribution, metabolism and/or excretion, rise, cost of side effects treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0047] compound
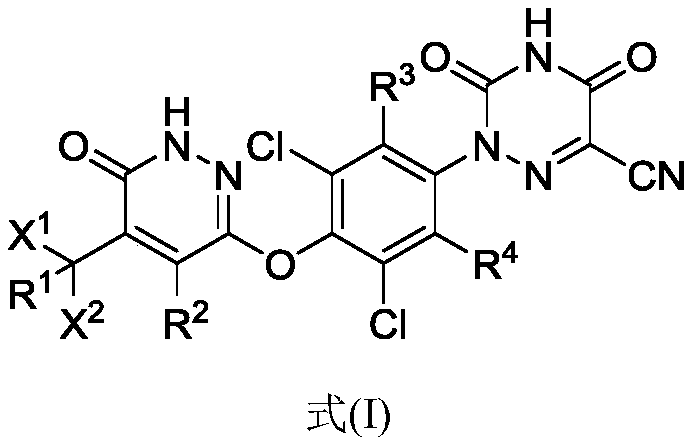
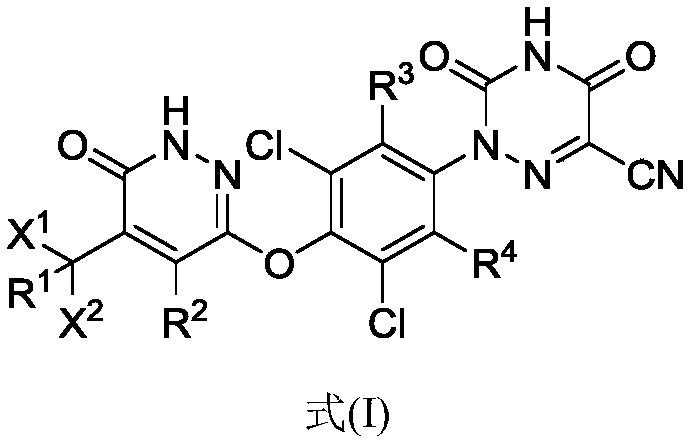
[0048] The present invention provides a compound of formula (I), or a pharmaceutically acceptable salt, prodrug, hydrate or solvate, crystal form, stereoisomer or isotopic variant thereof:
[0049]
[0050] in,
[0051] R 1 , R 2 , R 3 and R 4 each independently selected from hydrogen or deuterium;
[0052] x 1 and x 2 each independently selected from CH 3 、CD 3 、CHD 2 or CH 2 D;
[0053] The condition is that if X 1 and x 2 Both are CH 3 , then R 1 , R 2 , R 3 and R 4 At least one of is deuterium.
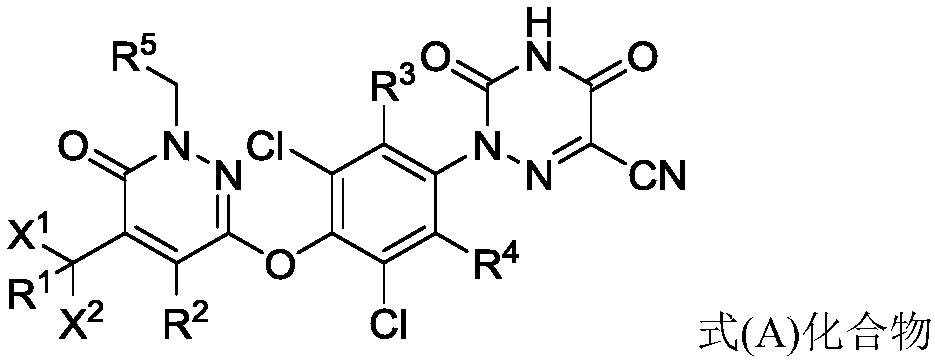
[0054] As a preferred embodiment of the present invention, the compound in formula (I) contains at least one deuterium atom, more preferably one deuterium atom, more preferably two deuterium atoms, more preferably three deuterium atoms, more preferably four deuterium atoms , more preferably five deuterium atoms, more preferably six deuterium atoms, more preferably seven deuterium atoms, more preferably eight deuterium atoms, more preferably...
Embodiment 1
[0138] Example 1 2-(3,5-dichloro-4-((6-oxo-5-(propan-2-yl-1,1,1-d 3 )-1,6-dihydropyridazine-3- Preparation of (yl)oxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile (compound M-1).
[0139]
[0140] Synthesize using the following route:
[0141]
[0142]
[0143] Synthesis of Step 1 Compound 2
[0144] Compound 1 (5.5g, 31.6mmol) and sodium ethoxide (2.6g, 37.9mmol) were added to ethanol (30ml) in turn, and the solution was stirred at 70°C for 0.5h, cooled to room temperature, and deuterated iodomethane ( 5.0g, 34.7mmol) was slowly added dropwise to the above solution, the reaction solution was stirred at room temperature for 20h, most of the solvent was removed, the residual solution was extracted with dichloromethane (40ml×2), the organic phases were combined, anhydrous sodium sulfate After drying, the solvent was removed to obtain 5.1 g of a brownish-red oil, with a yield of 85%.
[0145] Synthesis of Step 2 Compound 3
[0146] Potassium ...
Embodiment 2
[0159] Example 2 2-(3,5-dichloro-4-((6-oxo-5-(propan-2-yl-1,1,1,3,3,3-d 6 )-1,6-dihydro Pyridazin-3-yl)oxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile (compound M-2 ) system prepare.
[0160]
[0161] Synthesize using the following route:
[0162]
[0163] Synthesis of step 1 compound 13
[0164] Compound 12 (4.0g, 25.0mmol) and sodium ethoxide (4.0g, 57.5mmol) were added to ethanol (30ml) in sequence, the solution was stirred at 70°C for 0.5h, cooled to room temperature, and deuterated iodomethane ( 8.0g, 55.0mmol) was slowly added dropwise to the above solution, the reaction solution was stirred at room temperature for 20h, most of the solvent was removed, the residual solution was extracted with dichloromethane (50ml×2), the organic phases were combined, anhydrous sulfuric acid Dry over sodium, and remove the solvent to obtain 3.8 g of brown-red oily substance, yield 78%.
[0165] Synthesis of Step 2 Compound 14
[0166] Potassium hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| rate of recovery | aaaaa | aaaaa |
| rate of recovery | aaaaa | aaaaa |
| rate of recovery | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com