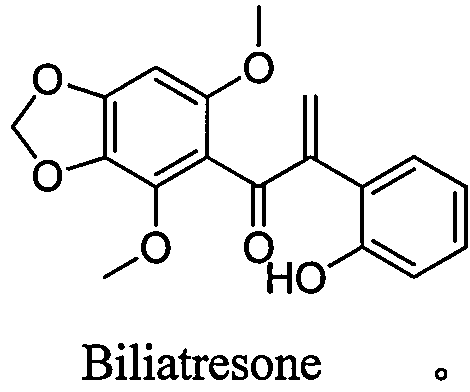
Biliatresone, as well as preparation method, medicinal composition and application thereof
A composition and compound technology, applied in the field of medicine, can solve the problems of limiting the in-depth study of animal models of biliary atresia, the single origin of raw materials of Biliatresone, and the slow progress in the exploration of the pathogenesis and prevention methods of biliary atresia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
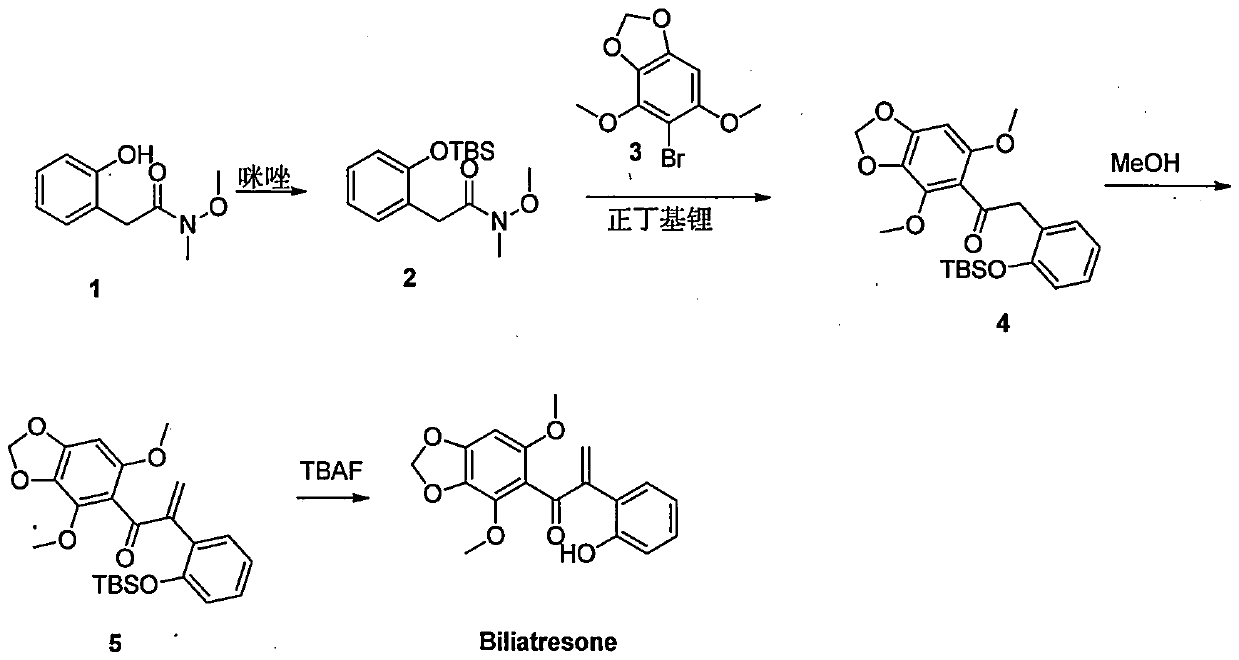
[0025] Example 1 Synthesis of Biliatresone Compounds
[0026] According to the following synthetic route:
[0027]
[0028] The first step: TBSCl (4.3g, 28.5mmol) dissolved in 10 milliliters of DCM was added dropwise to compound 1 (synthetic references Clarke, Aimee K.; James, Michael J.; etal.Angewandte Chemie- International Edition, 2016, vol.55, #44p.13798-13802) (3.7g, 18.9mmol) and 70ml of DCM solution of imidazole (2.59g, 38mmol), the addition was completed in about 30 minutes, and it rose naturally to Reacted at room temperature overnight, TLC raw materials reacted completely, filtered, the filtrate was washed with water, separated, the organic phase was dried and passed through the column to obtain the product compound 2, 5 g, the yield was 85%, and the appearance was a colorless oil;
[0029] The second step: compound 3 (540mg, 2.06mmol) (synthetic references Shuji Jinno, TakaakiOkit, and Kuniyo Inouye, Bioorganic & Medicinal Chemistry Letters 9 (1999) 1029-1032) ...
Embodiment 2
[0032] Embodiment 2 animal experiments
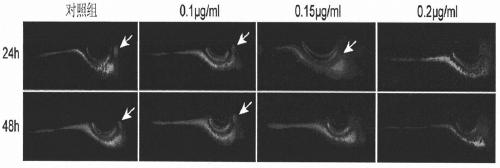
[0033] The compound Biliatresone, as a biliary epithelial drug, was used to induce biliary atresia in animal models.
[0034] Set up a concentration gradient of Biliatresone, respectively 0.1μg / ml, 0.15μg / ml, 0.2μg / ml, and the control group to treat the zebrafish larvae 5 days after fertilization for 24h and 48h, and feed the long-chain fatty acid Bodipy- C16 (0.2μg / ml), using a fluorescent microscope to photograph the development of the gallbladder and intestinal tract;
[0035]The results showed that as the concentration of Biliatresone increased and the treatment time increased, gallbladder development disorders appeared in zebrafish larvae, and the gallbladder of zebrafish larvae disappeared after 0.2 μg / ml Biliatresone treatment for 24 hours and 0.15 μg / ml Biliatresone treatment for 48 hours. The results confirmed that the prepared The compound Biliatresone is biologically active.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com