Mometasone furoate gelatin and preparation method thereof
A technology of mometasone furoate and gel, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of fast growth and product quality risks, and achieve growth Stability, good application feeling, and stable drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
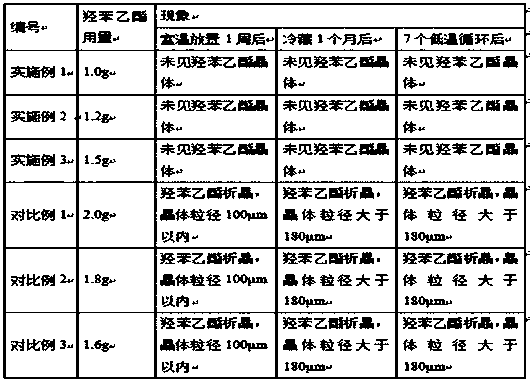
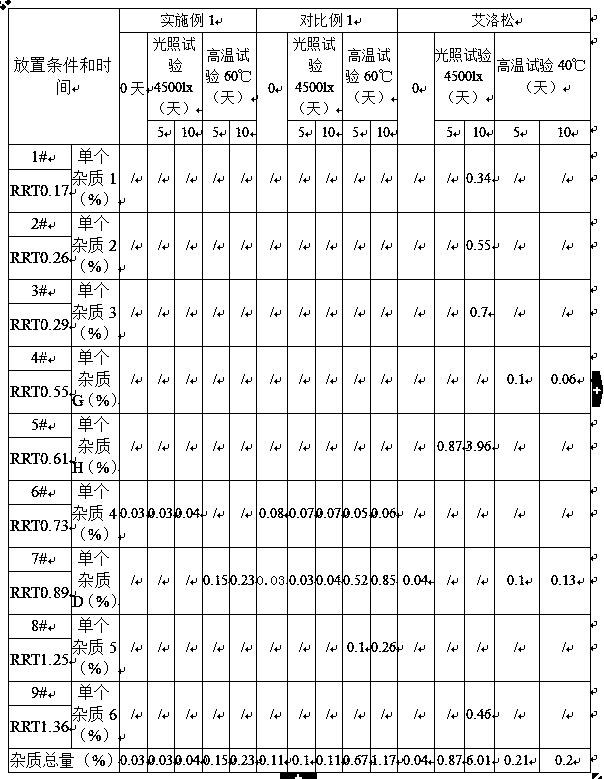
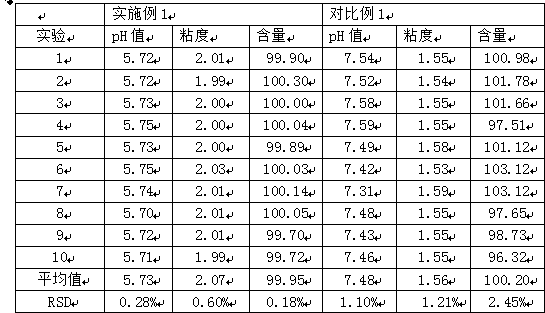
[0031] Embodiment 1: mometasone furoate gel, is characterized in that: be made of the raw and auxiliary materials of following weight ratio: mometasone furoate 1.0g, carbomer 10.0g, triethanolamine 5.0g, medicinal ethanol 50.0g , glycerin 150.0g, ethylparaben 1.0g, add purified water to a total weight of 1000g;
[0032] The preparation method of mometasone furoate gel is as follows: (1) Weigh the prescribed amount of carbomer and disperse it in 600g of purified water, fully swell for 3 hours; add glycerin and stir evenly; dilute with triethanolamine and the remaining amount of purified water, and stir (2) Mix mometasone furoate and ethylparaben in medicinal ethanol, stir to dissolve and heat to 55°C, Add it into the matrix under stirring, and continue to stir to obtain a milky white translucent gel, which is packaged.
Embodiment 2
[0033] Embodiment 2: mometasone furoate gel, is characterized in that: be made of the raw and auxiliary materials of following weight ratio: mometasone furoate 1.0g, carbomer 10.0g, triethanolamine 4.5g, medicinal ethanol 50.0g , glycerin 150.0g, ethylparaben 1.2g, add purified water to a total weight of 1000g;
[0034]The preparation method of mometasone furoate gel is as follows: (1) Weigh the prescribed amount of carbomer and disperse it in 600g of purified water, and fully swell for 3.5 hours; add glycerin and stir evenly; Add to the carbomer solution under stirring, and stir while adding to obtain a transparent gel matrix; (2) Mix mometasone furoate and ethyl paraben in medicinal ethanol, stir to dissolve and heat to 60°C, Add it into the matrix under stirring, and continue to stir to obtain a milky white translucent gel, which is packaged.
Embodiment 3
[0035] Embodiment 3: mometasone furoate gel, is characterized in that: be made of the raw and auxiliary materials of following weight ratio: mometasone furoate 1.0g, carbomer 10.0g, triethanolamine 4.0g, medicinal ethanol 50.0g , glycerin 150.0g, ethylparaben 1.5g, adding purified water to a total weight of 1000g, all the other with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com