Application of eleutheroside B in preparation of medicine for preventing or treating acute kidney injury
A technology for acute kidney injury and Acanthopanax senticosus, applied in the field of biomedicine, can solve problems such as unreported effects, and achieve the effects of reducing apoptosis and necrosis response, reducing inflammatory response, and reducing the level of inflammatory factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
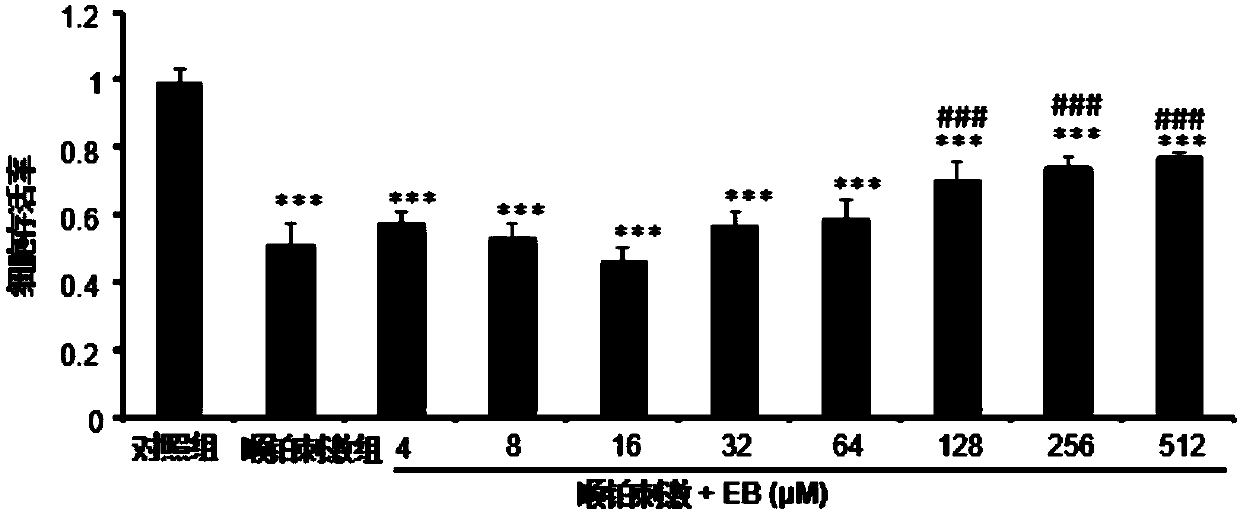
[0035] Protective effect of eleuthelis B (EB) on cisplatin (Cisplatin)-induced renal tubular epithelial cell injury in vitro.
[0036] MTT method: human renal tubular epithelial cells (HK2) cells were seeded in a 96-well plate at a seeding density of about 4000 cells / well. Cultured for 24 hours, after being starved for 12 hours with serum-free medium, they were divided into 8 groups, namely control group, cisplatin stimulation group and 8 eleutheroside B gradient dose groups (cisplatin stimulation + EB). 20 μM cisplatin was added to the group, and 20 μM cisplatin + 4 μM EB, 20 μM cisplatin + 8 μM EB, 20 μM cisplatin + 16 μM EB, 20 μM cisplatin + 32 μM EB, 20 μM cisplatin were added in the 8 eleutheroside B gradient dose groups. Platinum + 64 μM EB, 20 μM cisplatin + 128 μM EB, 20 μM cisplatin + 256 μM EB, 20 μM cisplatin + 512 μM EB, and continue to culture for 24 hours. After the incubation, 20 μL of 5 g·L-1 MTT solution was added to each well, and the incubation was continu...
Embodiment 2
[0042] The inhibitory effect of eleuthelis B on cisplatin-induced renal tubular epithelial cell inflammatory factors (TNF-α and IL-6) in vitro.
[0043] HK2 cells were seeded in 12-well plates at a seeding density of about 0.5×105 cells / well, and were divided into control group, cisplatin stimulation group, eleutheroside B group (EB) and cisplatin stimulation + EB group, Repeat the experiment 3-4 times for each group, incubate for 24 hours and starve for 12 hours with serum-free medium, then add 20 μM cisplatin in the model group, add eleutheroside B group (EB) and cisplatin + EB group respectively 128 μM eleutheroside B, continued to culture for 24 hours, washed three times with PBS, collected cells, extracted RNA, reverse transcribed, and amplified. Then Real time PCR was carried out to detect the expression levels of tumor necrosis factor (TNF-α) and interleukin-6 (IL-6), and the results were as follows: Figure 4 (TNF-α) and Figure 5 (IL-6).
[0044] Obtainable by this...
Embodiment 3
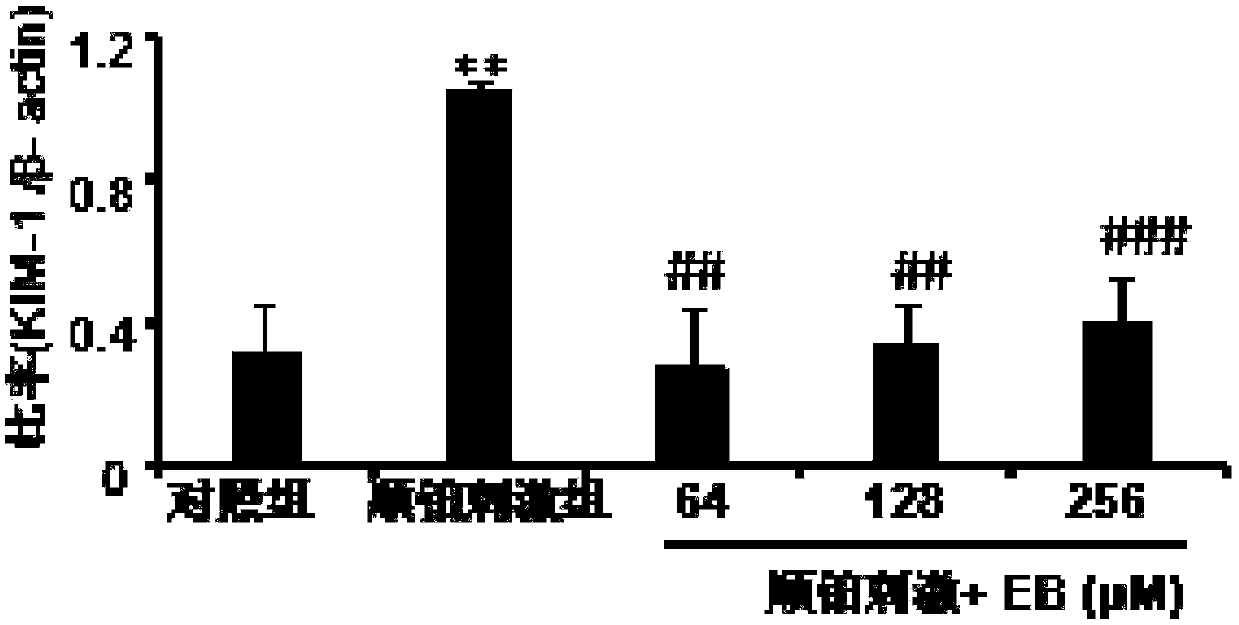
[0047]In vitro, eleuthelis B can inhibit the apoptosis and necrosis of renal tubular epithelial cells induced by cisplatin.
[0048] Western Blot: HK2 cells in the logarithmic growth phase were inoculated into 6-well plates at a seeding density of about 1.0×105 cells / ml, and were divided into control group, cisplatin stimulation group, and eleutheroside B group (EB) , cisplatin stimulation + eleutheroside B group (cisplatin stimulation + EB), add 20 μM cisplatin in the cisplatin stimulation group, add 20μM Cisplatin+128μM EB, repeat the experiment 3-4 times for each group, continue to culture for 24 hours, wash three times with PBS, collect the cells, extract the total protein, detect the changes in the expression levels of apoptosis-related proteins RIPK1 and RIPK3 by Western Blot, and Semi-quantitative analysis of RIPK1 and RIPK3 (eg Figure 6 and Figure 7 shown).
[0049] Obtainable by this embodiment:
[0050] Figure 6 and 7 The results of Western Blot and semi-qua...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com