Oral solid tablet containing metronidazole and preparation method thereof
A technology containing metronidazole and metronidazole, which is applied in pill delivery, pharmaceutical formulations, coatings, etc., can solve the problems of increasing solubility, and no improvement in the intra-batch variation of dissolution curves, etc., to achieve definite curative effect, good uniformity, The effect of simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
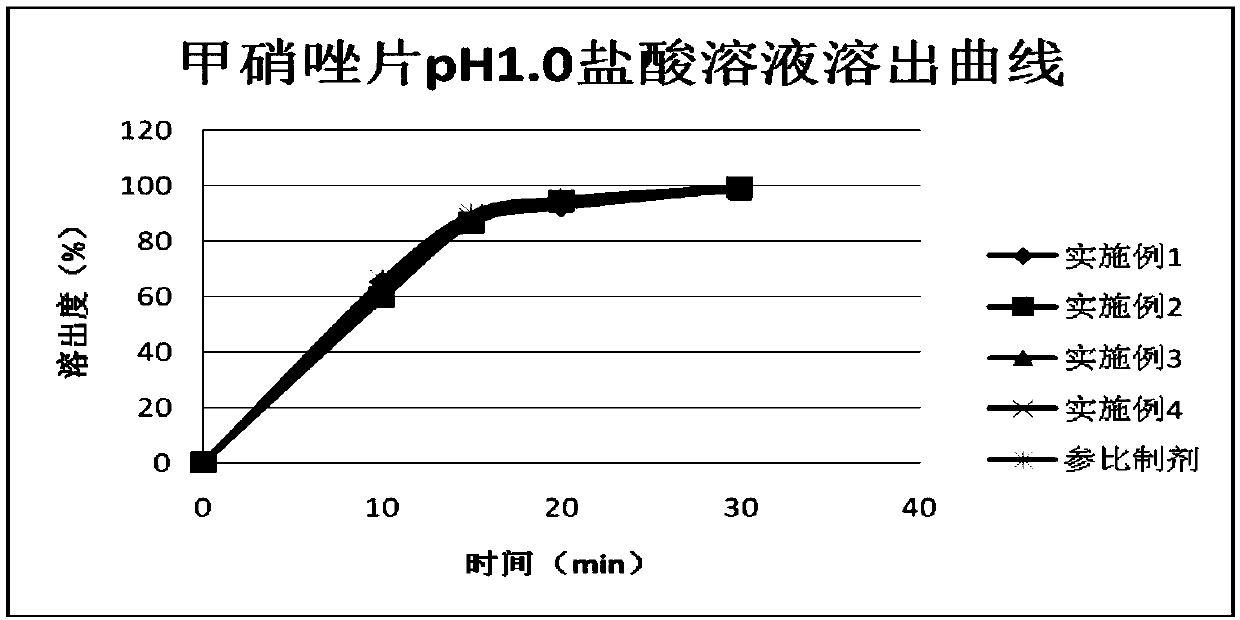
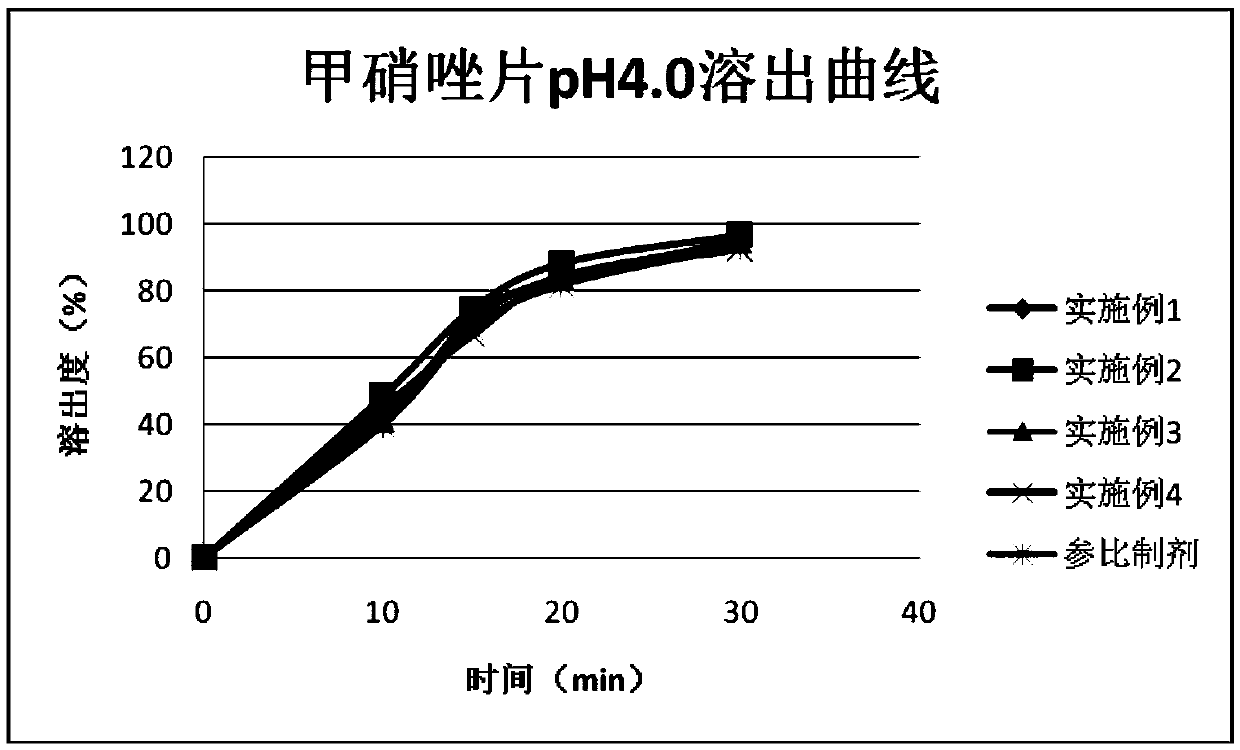
Embodiment 1
[0043] A metronidazole-containing oral solid tablet of the present embodiment comprises a drug tablet core and a coating layer, wherein: the drug tablet core is composed of the following component raw materials:
[0044]
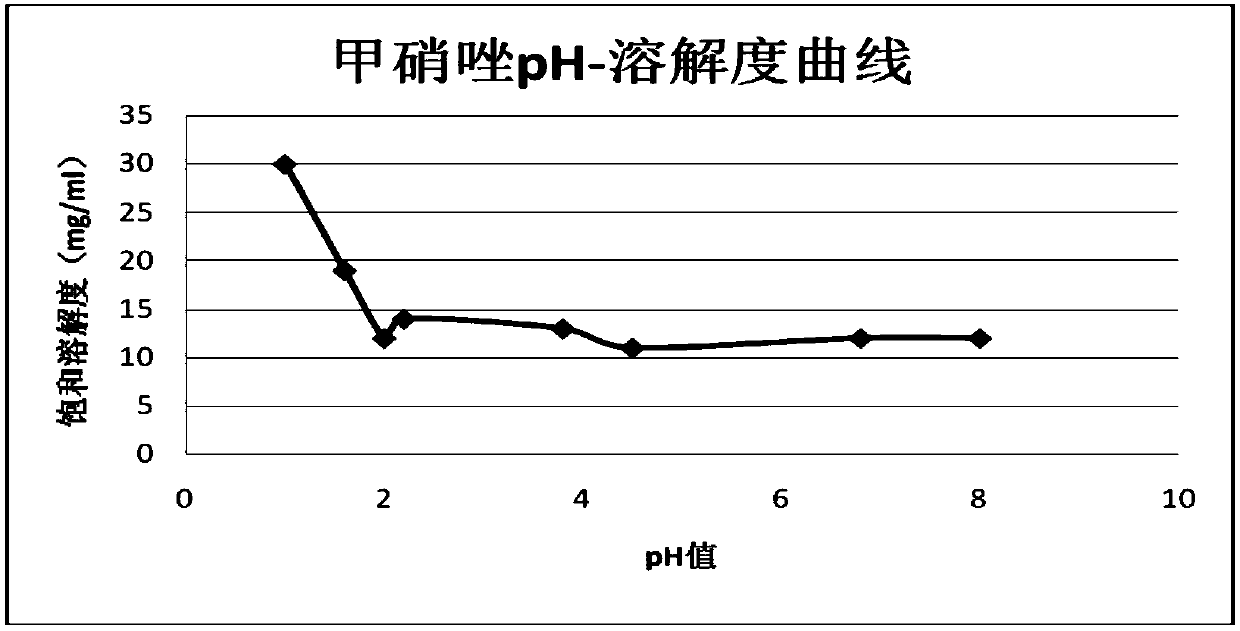
[0045]Wherein: the filler is composed of 94g cornstarch and 24g calcium hydrogen phosphate; the particle size of the metronidazole is D50≤40μm, D90≤90μm; the particle size distribution of each component raw material of the drug tablet core after total mixing As follows: 20-60 mesh particles account for 50-70%, 60-100 mesh particles account for 10-30%, and fine powder below 100 mesh accounts for 5-20% (see Table 1 and Table 2 for specific particle size distribution) .
[0046] The coating layer consists of:
[0047] Opadry Gastric Film Coating Premix: 10g.
[0048] The preparation method of the above-mentioned oral tablet containing metronidazole is as follows: the metronidazole is crushed to a particle size of D50≤40 μm, D90≤90 μm; then mixed with corn ...
Embodiment 2
[0050] A metronidazole-containing oral tablet of the present embodiment includes a drug tablet core and a coating layer, wherein: the drug tablet core is composed of the following component raw materials:
[0051]
[0052] Wherein: the metronidazole particle size is D50≤40μm, D90≤90μm; the particle size distribution of the raw materials of the various components of the drug tablet core is mixed as follows: 20-60 mesh particles account for 50-70%, 60 ~100 mesh particles accounted for 10~30%, and fine powder below 100 mesh accounted for 5~20% (see Table 1 and Table 2 for specific particle size distribution).
[0053] The coating layer consists of:
[0054] Opadry Gastric Film Coating Premix: 10g.
[0055] The preparation method of the above-mentioned oral tablet containing metronidazole is as follows: the metronidazole is pulverized to a particle size of D50≤40 μm, D90≤90 μm; After mixing Ketone K30, add purified water to granulate, dry, and granulate into granules with a p...
Embodiment 3
[0057] A metronidazole-containing oral tablet of the present embodiment includes a drug tablet core and a coating layer, wherein: the drug tablet core is composed of the following component raw materials:
[0058]
[0059] Wherein: the metronidazole particle size is D50≤40μm, D90≤90μm; the particle size distribution of the raw materials of the various components of the drug tablet core is mixed as follows: 20-60 mesh particles account for 50-70%, 60 ~100 mesh particles accounted for 10~30%, and fine powder below 100 mesh accounted for 5~20% (see Table 1 and Table 2 for specific particle size distribution).
[0060] The coating layer consists of:
[0061] Opadry Gastric Film Coating Premix: 10g.
[0062] The preparation method of the above-mentioned oral tablet containing metronidazole is as follows: the metronidazole is crushed to a particle size of D50≤40 μm, D90≤90 μm; then mixed with pregelatinized starch, microcrystalline cellulose, croscarmell Sodium cellulose and hi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com