Solubleand efficient recombinant expression methodfor fibroblast growth factor protein
A technology for fibroblasts and growth factors, applied in the field of recombinant proteins, can solve the problems of difficulty in obtaining efficient and stable expression of fibroblast growth factor proteins, and achieve the effects of being conducive to popularization and application, good prospects and economic value, and a simple method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
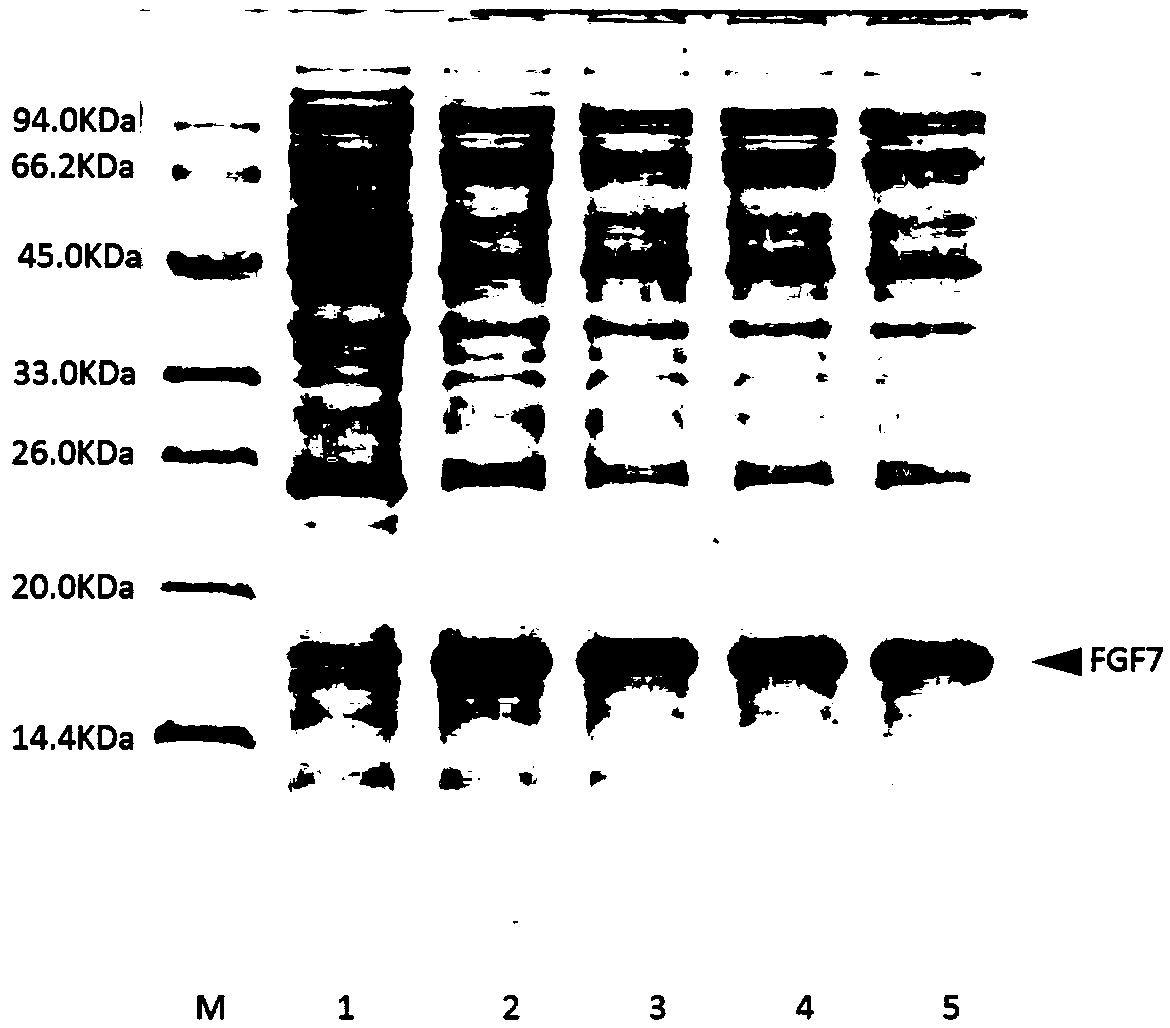
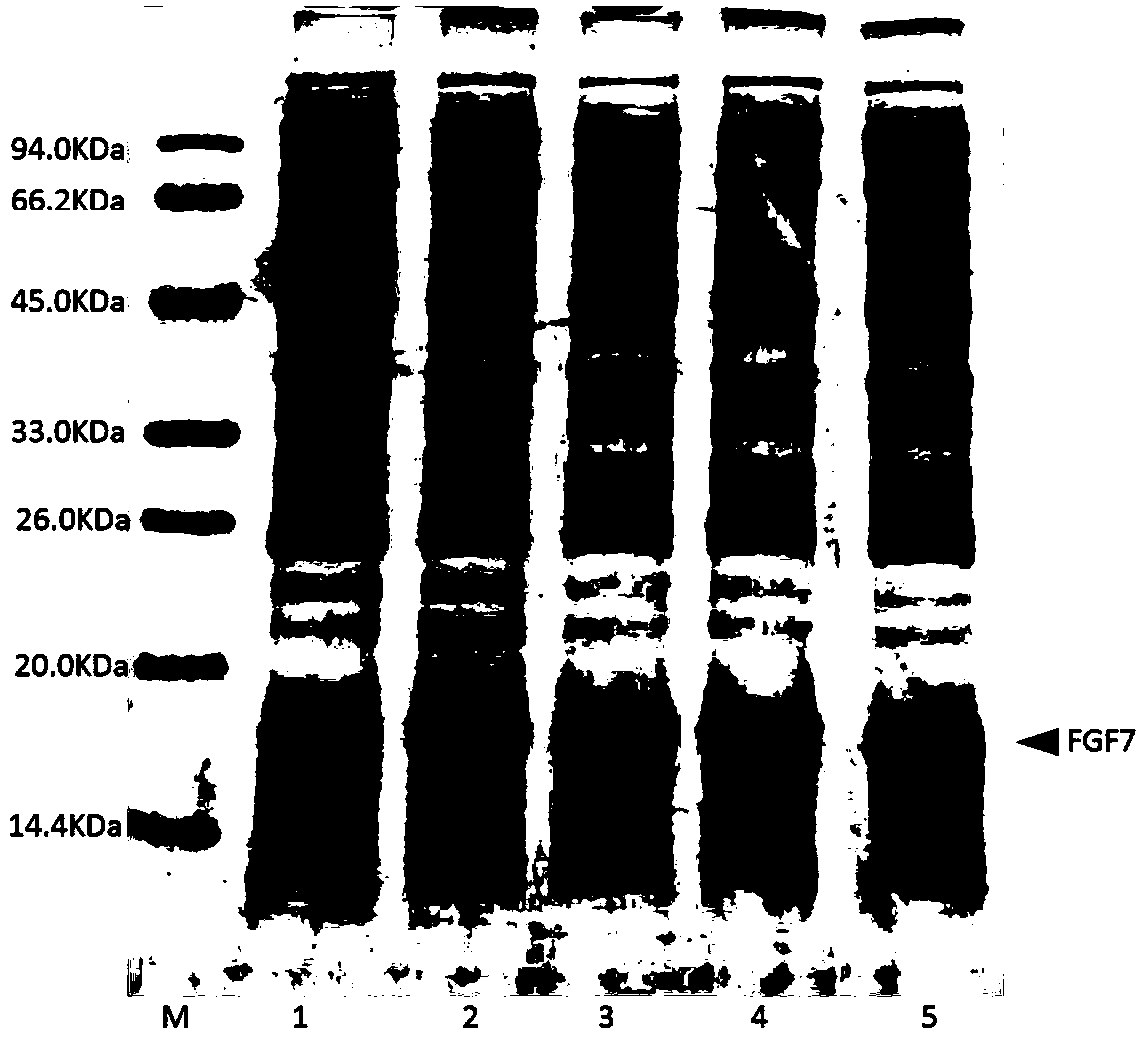
[0042] Example 1: Construction of recombinant FGF7 expression strain.
[0043] According to the human FGF7 amino acid sequence provided by the NCBI database (sequence SEQ ID NO: 1), and combined with the E. coli code preference principle, the cDNA sequence encoding the amino acid of the FGF7 variant was designed, and the FGF7 variant containing the coded FGF7 variant was obtained through full gene synthesis DNA fragments. The DNA fragment encoding the FGF7 variant obtained from the whole gene, and the pET-3c vector plasmid, which is the expression vector regulated by the T7 promoter, were digested with Nde I+BamH I and ligated by DNA polymerase to obtain Circular recombinant plasmid encoding FGF7 variant. Transform the obtained recombinant plasmid into BL21(DE3)plysS competent cells, spread it on an LB agarose plate containing 100ug / ml ampicillin and 25ug / ml chloramphenicol resistance, and screen out monoclonal colonies to obtain expression recombinant The engineered strain of ...
Embodiment 2
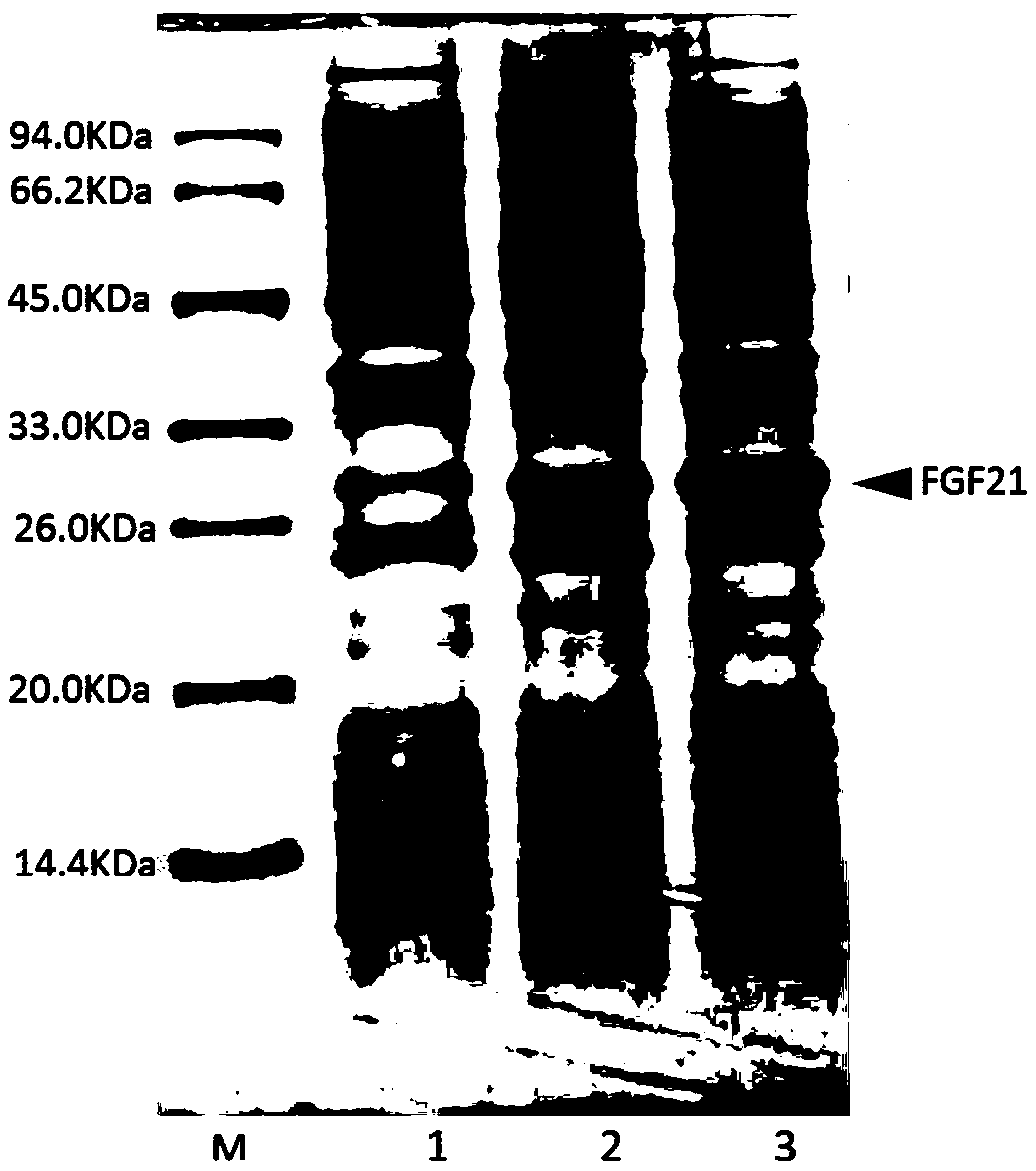
[0044] Example 2: Construction of recombinant FGF21 expression strain.
[0045] According to the human FGF21 amino acid sequence (SEQ ID NO: 2) provided by the NCBI database, combined with the E. coli code preference principle, a cDNA sequence encoding FGF21 amino acid was designed, and a DNA fragment encoding FGF21 was obtained through full gene synthesis. The DNA fragment encoding FGF21 obtained from the whole gene, and the pET-30a vector plasmid, which is a T7 promoter-regulated expression vector, were digested with Nde I+BamH I and ligated by DNA polymerase to obtain FGF21 The circular recombinant plasmid. The obtained recombinant plasmid pET-30a-FGF21 plasmid was transformed into BL21(DE3)plysS competent, and spread on LB agarose plate containing 50ug / ml kanamycin and 25ug / ml chloramphenicol resistance, and screened out Monoclonal colonies were used to obtain engineered bacteria expressing recombinant FGF21, named pET-30a-FGF21 / BL21(DE3)plysS.
Embodiment 3
[0046] Example 3: Construction of recombinant FGF18 expression strain.
[0047] According to the human FGF18 amino acid sequence provided by the NCBI database (SEQ ID NO: 3), combined with the E. coli code preference principle, a cDNA sequence encoding FGF18 variant amino acids was designed, and a DNA fragment encoding FGF18 was obtained through full gene synthesis . The DNA fragment encoding FGF18 obtained from the whole gene, and the pET-30a vector plasmid, which is a T7 promoter-regulated expression vector, were digested with Nde I+BamH I and ligated by DNA polymerase to obtain a DNA fragment containing encoding FGF18 Variant circular recombinant plasmid. The obtained recombinant plasmid pET-30a-FGF18 plasmid was transformed into BL21(DE3) competent, and spread on the LB agarose plate containing 50ug / ml kanamycin and 25ug / ml chloramphenicol resistance, and the single The colony was cloned to obtain an engineered strain expressing recombinant FGF18, named pET-30a-FGF18 / BL21(D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com