Methods for selectively modulating the activity of distinct subtypes of cells
A target cell, cell targeting technology, applied in the active field of different subtypes, can solve the problem of expensive culture system, achieve the effect of improving efficiency and preventing in vivo resistance problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
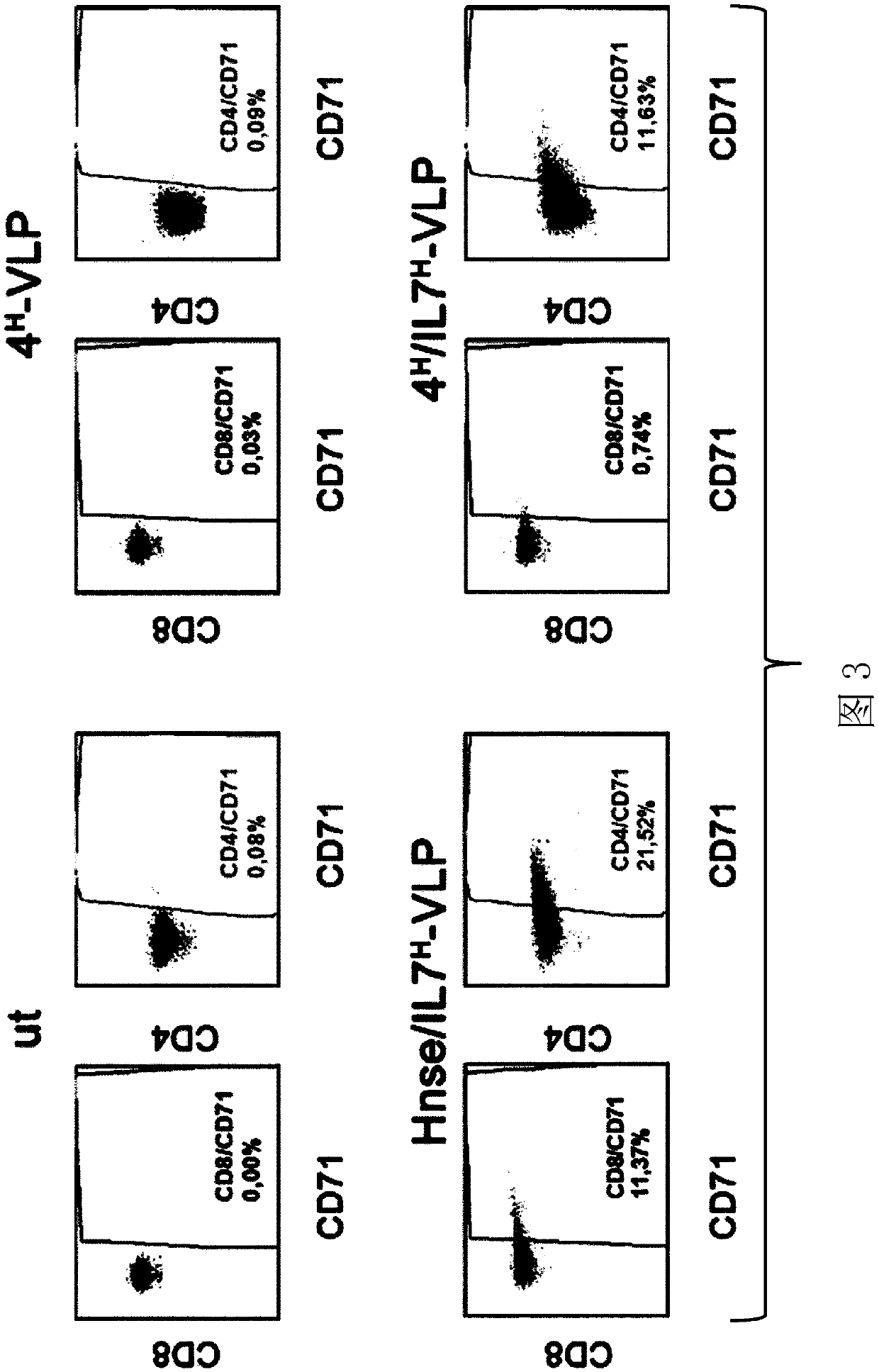
[0432] Example 1: Selective activation of CD4+ T cells
[0433] To activate CD4 + Lymphocytes do not activate CD8 + Lymphocytes, generated VLPs that presented CD4-specific DARPins as targeting ligands and IL-7 as activation domains on the MV H protein as well as the fusion protein (F), resulting in 4 H / IL7 H - VLPs (proteins comprising the sequences SEQ ID NO: 2, 14 and 15). To generate particles, a transfection protocol was established. Briefly, 0.45 μg of pCG-Hmut-CD4-DARPin (Zhou et al., J Immunol, 2015), 0.45 μg of pCG-HΔ15-IL7 (provided by Els Verhoeyen), 4.7 μg of pCG-FcΔ30 (Funke et al., Molecular therapy, 2008), 14.4 μg of HIV-1 packaging plasmid pCMVΔR8.9 (Funke et al., Molecular therapy, 2008) and 15.1 μg of pCG-1 were transfected into HEK293T cells in a T175 flask. After harvesting, the carrier particles are further concentrated by ultracentrifugation. A titer of ~10 LV was obtained 7 tu / ml, a VLP titer of ~1.5 μg p24 / ml was obtained.
[0434] 4 was confi...
Embodiment 2
[0436] Example 2: Delivery of tumor-specific chimeric antigen receptors into resting T cells
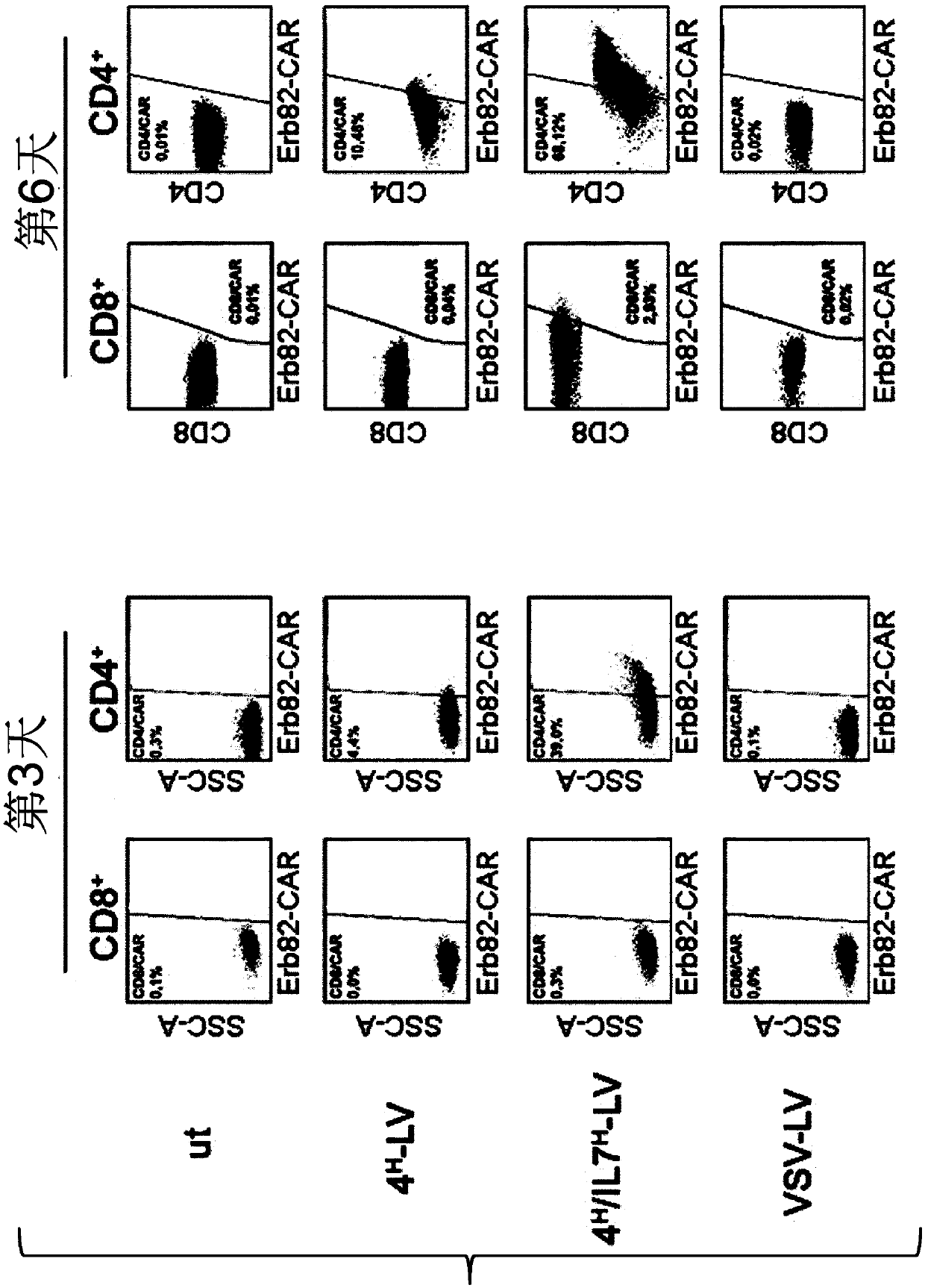
[0437] Since IL7-presenting vectors trigger activation of resting T cells, these cells are expected to allow lentiviral transduction. To demonstrate this, two versions of the 4 H / IL7 H -LV delivery of the GFP transgene. Such as Figure 9 shown, 4 H / IL7 HΔ15 -LV and 4 H / IL7 HΔ20 -LVs can efficiently and selectively transduce resting CD4 in cell mixtures + T cells.
[0438] Chimeric antigen receptors (CARs) are powerful tools for cancer therapy. Until now, for the genetic delivery of CARs, T cell subsets had to be purified and activated. Here, it is demonstrated that CAR can be selectively delivered to resting CD4 + in cells. The particles were generated as described in Example 1, except that pCG-1 was replaced by a CAR-encoding transfer plasmid. When resting T cells are delivered with 4 H / IL7 H - When transduced with ErbB2-specific chimeric antigen receptor (ErbB2...
Embodiment 3
[0439] Example 3: MV glycoproteins can be replaced by NiV's MV glycoproteins to selectively activate and transduce resting human CD8 + T cells
[0440] To efficiently pseudotype VLPs or LVs with NiV glycoproteins, the cytoplasmic tail of NiV-G was truncated by 34 amino acids (GcΔ34) and that of NiV-F by 22 amino acids (FΔ22). Next, the natural receptor recognition of Ephrin-B2 / B3 by GcΔ34 was disrupted by introducing four point mutations (E501A, W504A, Q530A, E533A) into GcΔ34. LVs pseudotyped with this engineered G protein completely lost binding to native Ephrin-B2 and Ephrin-B3NiV receptors ( figure 2 ), and do not enter cells expressing these receptors.
[0441] Furthermore, NiV-pseudotyped LVs have some attractive features, such as high yield and resistance to intravenous immunoglobulin. Since there is no vaccination against NiV and the few outbreaks are limited to a few cases in Malaysia, Bangladesh and India (SEARO|WHO Southeast Asia Region), neutralizing antibodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com