Stable insulin analogue injection and preparation method thereof
A technology for insulin analogues and injections, which is applied in the field of medicine, can solve problems such as oxidative damage and shock, and achieve the effects of improved stability, simple preparation process, prevention of oxidative damage, and even changes in the color of the liquid medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
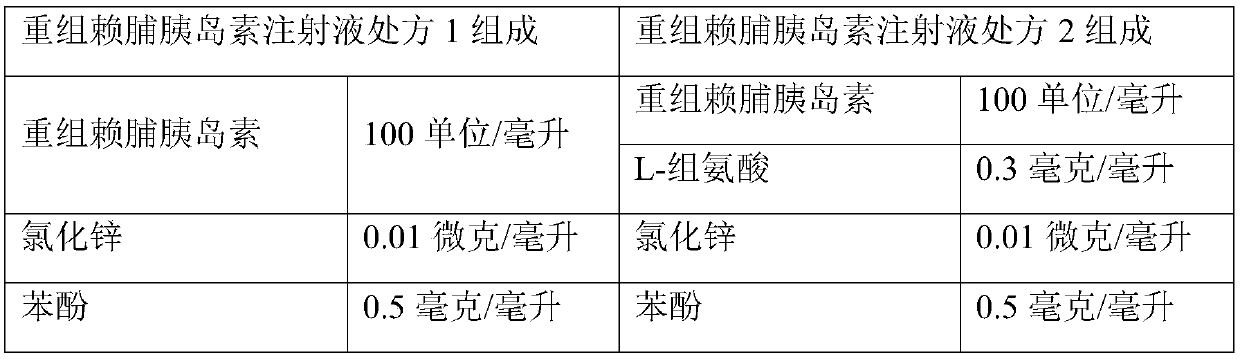
[0026] Preparation of Recombinant Insulin Lispro Injection
[0027] Prepare the injection according to the following formula:
[0028]
[0029]
[0030] The specific preparation method is as follows:
[0031] Recombinant insulin lispro injection prescription 1 preparation process:
[0032] (1) Add phenol, m-cresol, glycerin, disodium hydrogen phosphate, and zinc chloride into water for injection, stir until completely dissolved, and obtain solution ①;
[0033] (2) Add the recombinant insulin lispro into the water for injection, add a pH regulator to adjust the pH value of the liquid to 2.8-3.2, stir until clear to obtain a solution, add solution ② to obtain solution ③.
[0034] (3) Add the solution ① into the solution ③ under the condition of stirring, mix evenly, add a pH regulator to adjust the pH value of the medicinal solution to 7.0-7.8, constant volume, filter and sterilize to obtain the product.
[0035] Preparation process of Recombinant Insulin Lispro Injecti...
Embodiment 2
[0053] Preparation of recombinant insulin glargine injection
[0054] Prepare the injection according to the following formula:
[0055]
[0056]
[0057] The specific preparation method is as follows:
[0058] Recombinant insulin glargine injection prescription 1 preparation process:
[0059] (1) Add m-cresol, glycerin, and zinc chloride to water for injection, stir until completely dissolved, and obtain solution ①;
[0060] (2) Add the recombinant insulin glargine into the water for injection, add a pH regulator to adjust the pH value of the liquid to 3.0-4.0, stir until clear to obtain a solution, and obtain solution ②.
[0061] (3) Add the solution ① into the solution ② under the condition of stirring, mix well, adjust the pH value to 3.5-4.5, constant volume, filter and sterilize to obtain the product.
[0062] Recombinant insulin glargine injection prescription 2 preparation process
[0063] (1) Add m-cresol, glycerin, and zinc chloride to water for injection, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com