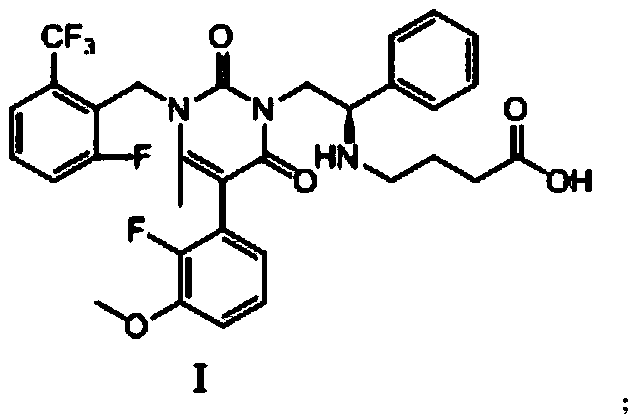
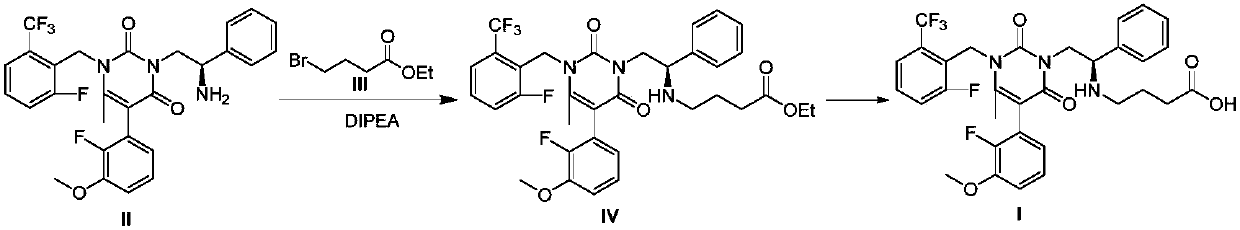
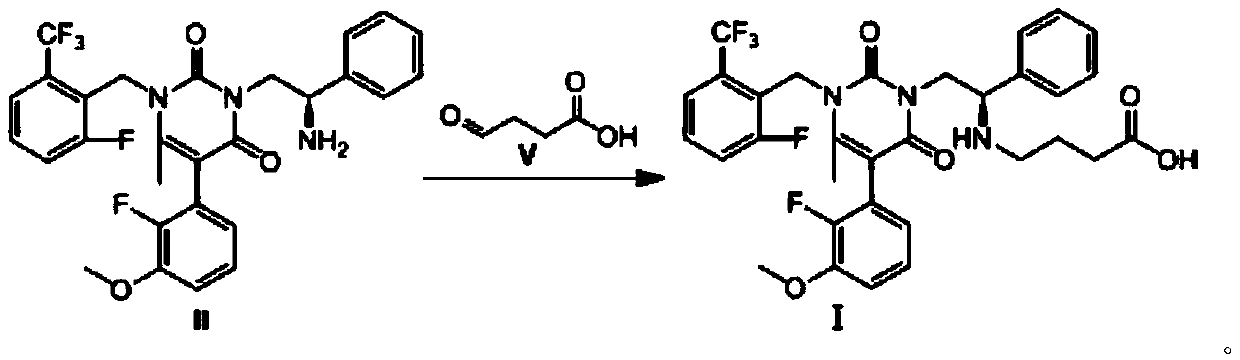
Preparation method of Elagolix
An amino and phenethyl technology, applied in the field of Elagolix preparation, can solve the problems of long Elagolix route and difficult purification, and achieve the effect of simple and convenient purification treatment, improved purity and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In the reactor, 1.0 g, 1.0 equiv. of (R)-3-(2-amino-2-phenethyl)-5-(2-fluoro-3-methoxybenzene represented by formula II Base)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-6-methylpyrimidine-2,4(1H,3H)-dione was dissolved in 10 mL of dichloromethane, and 1.2 Equiv. 4-oxobutanoic acid shown in formula V, 1.0equiv. acetic acid and 3.0equiv. sodium triacetoxyborohydride, stirred and reacted at 25°C for 18h; , and saturated brine to wash the reaction solution, then dried with sodium sulfate and spin-dried the solvent, and finally obtained 0.65 equiv. Elagolix as shown in formula I by column chromatography, and its yield was 65%. Using high-resolution mass spectrometry (ESI-) detection, the detection value is 630.2039; M-H + The calculated value of the high-resolution mass spectrum is 630.2033, and the structure of the product can be confirmed by comparison.
Embodiment 2
[0027] In the reactor, 1.0 g, 1.0 equiv. of (R)-3-(2-amino-2-phenethyl)-5-(2-fluoro-3-methoxybenzene represented by formula II Base)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-6-methylpyrimidine-2,4(1H,3H)-dione was dissolved in 15 mL of dichloromethane, and 1.5 Equiv. 4-oxobutanoic acid shown in formula V, 2.0equiv. acetic acid and 3.0equiv. sodium cyanoborohydride, stirred and reacted at 25°C for 18h; The reaction solution was washed with brine, then dried with sodium sulfate and spin-dried to dry the solvent, and finally obtained 0.62 equiv. Elagolix as shown in formula I by column chromatography, and the yield was 62%. It was detected by high-resolution mass spectrometry (ESI-), and the detected value was 630.2036.
Embodiment 3
[0029] In the reactor, 1.0 g, 1.0 equiv. of (R)-3-(2-amino-2-phenethyl)-5-(2-fluoro-3-methoxybenzene represented by formula II Base)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-6-methylpyrimidine-2,4(1H,3H)-dione was dissolved in 12 mL of 1,2-dichloroethane, Add 1.0 equiv. of 4-oxobutanoic acid represented by formula V, 1.0 equiv. hydrochloric acid and 1.0 equiv. of sodium triacetoxy borohydride, and stir the reaction at 0°C for 6 h; after the reaction, The reaction solution was washed successively with 1M aqueous hydrochloric acid solution and saturated brine, then dried with sodium sulfate and spin-dried to dry the solvent, and finally 0.41 equiv. Elagolix as shown in formula I was obtained by column chromatography, and the yield was 41%. It was detected by high-resolution mass spectrometry (ESI-), and the detected value was 630.2036.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com