Sulfonamide derivative as well as preparation method and application thereof
A technology of derivatives and sulfonamides, applied in the field of sulfonamide derivatives and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
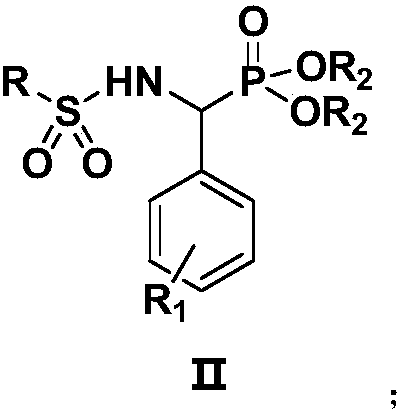
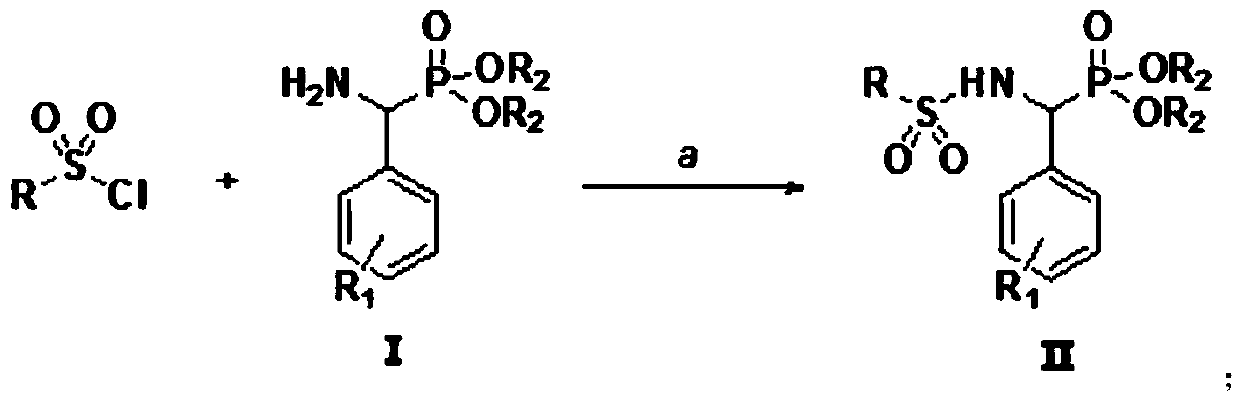
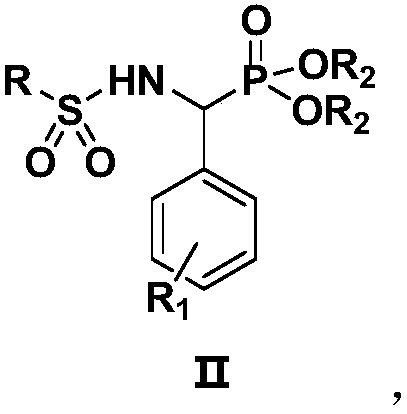
[0025] Preparation of N-[(diethoxyphosphono)-benzyl]-benzenesulfonamide (IIa):
[0026] Weigh 1.0mmol of intermediate 1 (diethyl α-aminobenzylphosphonate) and 1.0mmol of benzenesulfonyl chloride in a 50mL reaction flask, then add 10mL of dichloromethane and 0.5mmol of basic ionic liquid [Bmim]OH, Heated to reflux, TLC monitored the reaction, after 3 hours of reaction, the solvent was concentrated by a rotary evaporator, separated and purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:2), and a white solid was obtained, which was the target compound (Ⅱa). Yield 80.6%. compound 1 H NMR, 13 C NMR and MS: 1 H-NMR (400MHz, CDCl 3 )δ:6.98-7.72(m,11H,ArH+NH),4.78-4.87(q,1H,J=36.0Hz,PCH),4.25-4.28(m,2H,OCH 2 ),3.78-3.84(m,1H,OCH 2 ),3.49-3.57(m,1H,OCH 2 ),1.34(t,3H,J=16.0Hz,CH 3 ),0.98(t,3H,J=12.0Hz,CH 3 ). 13 C-NMR (100MHz, CDCl 3 )δ: 16.0, 16.4, 55.0, 63.6, 64.1, 126.4, 127.1, 127.2, 127.4, 127.5, 128.3, 128.6, 129.0, 129.1, 132.3, 135.6, 138.1. ES...
example 1
[0027] The above example 1, if according to the following method:
[0028] Weigh 1.0mmol of intermediate 1 (diethyl α-aminobenzylphosphonate) and 1.0mmol of benzenesulfonyl chloride in a 50mL reaction flask, then add 10mL of dichloromethane and 1.0mmol of triethylamine, heat to reflux, and monitor by TLC After 13 hours of reaction, the solvent was concentrated by a rotary evaporator, separated and purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:2), and a white solid was obtained, which was the target compound (IIa), with a yield of 39.5%.
Embodiment 2
[0030] Preparation of N-[(diethoxyphosphono)-benzyl]-2-thiophenesulfonamide (Ⅱb)
[0031] Weigh 1.0mmol intermediate 1 (diethyl α-aminobenzylphosphonate) and 1.0mmol thiophene-2-sulfonyl chloride in a 50mL reaction flask, then add 10mL dichloromethane and 0.5mmol basic ionic liquid [Bmim ]OH, heated to reflux, and TLC monitored the reaction. After completion, the solvent was concentrated by a rotary evaporator, separated and purified by column chromatography (eluent was ethyl acetate:petroleum ether=1:2), and a white solid was obtained, which was the target compound (Ⅱb). , yield 84.3%. compound 1 H NMR, 13 C NMR and MS: 1 H-NMR (400MHz, CDCl 3 )δ: 7.46-7.53 (m, 1H, NH), 6.67-7.28 (m, 8H, ArH), 4.72-4.80 (q, 1H, J = 32.0Hz, PCH), 4.22-4.29 (m, 2H, OCH 2 ),3.81-3.87(m,1H,OCH 2 ),3.52-3.62(m,1H,OCH 2 ),1.33(t,3H,J=16.0Hz,CH 3 ),0.99(t,3H,J=16.0Hz,CH 3 ). 13 C-NMR (100MHz, CDCl 3 )δ: 16.0, 16.4, 54.7, 63.7, 64.0, 126.7, 127.9, 128.0, 128.1, 128.2, 131.3, 132.0, 133.3, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com