Compound preparation containing mesalazine and acetaminophen, and application thereof
A technology for paracetamol and compound preparations is applied in the field of compound preparations containing mesalazine and paracetamol to achieve the effects of enhancing therapeutic effect, relieving pain and ensuring effective dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
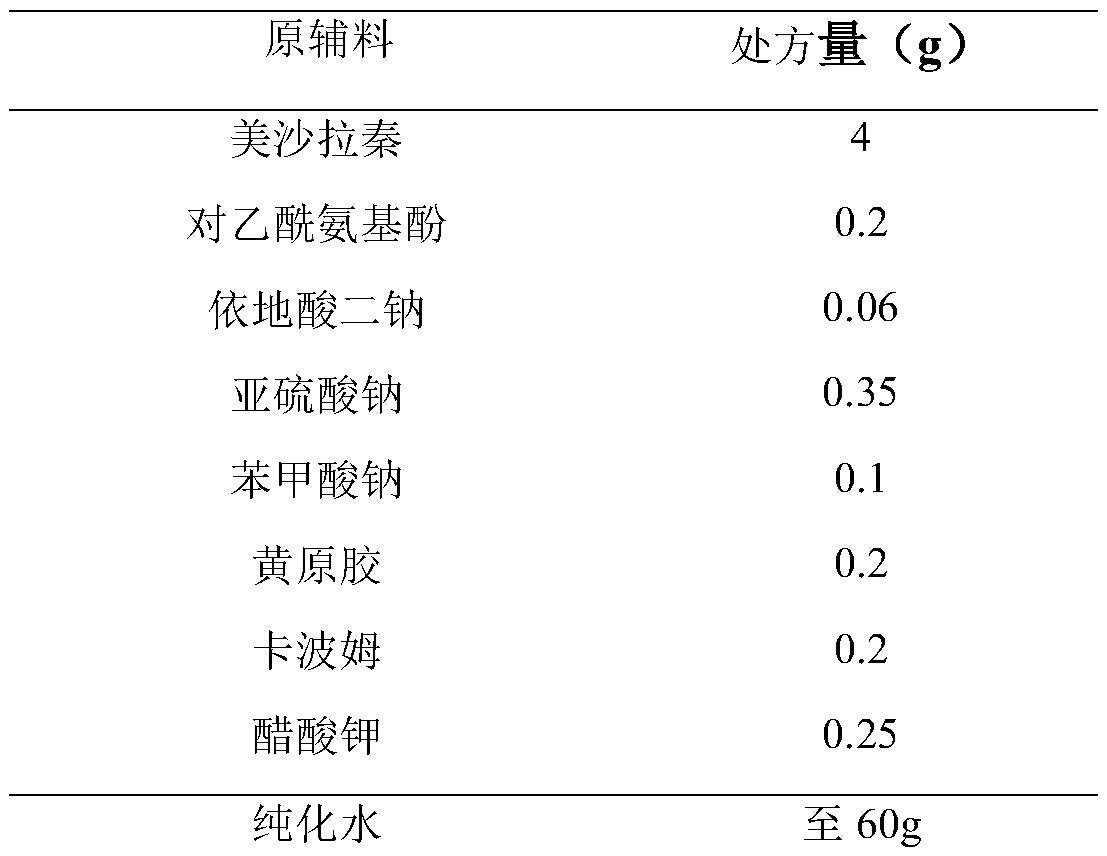
Embodiment 1
[0025]
[0026] Preparation Process:
[0027] (1) Get about 45g of new boiling water, add disodium edetate, sodium benzoate and potassium acetate of recipe quantity successively, stir until dissolved.
[0028] (2) Slowly add the xanthan gum and carbomer of the recipe amount under stirring, and stir to swell.
[0029] (3) Add sodium sulfite, mesalazine and acetaminophen in the prescribed amounts, stir and mix well.
[0030] (4) Homogenize, add new boiling water to the full amount, stir evenly, and fill.
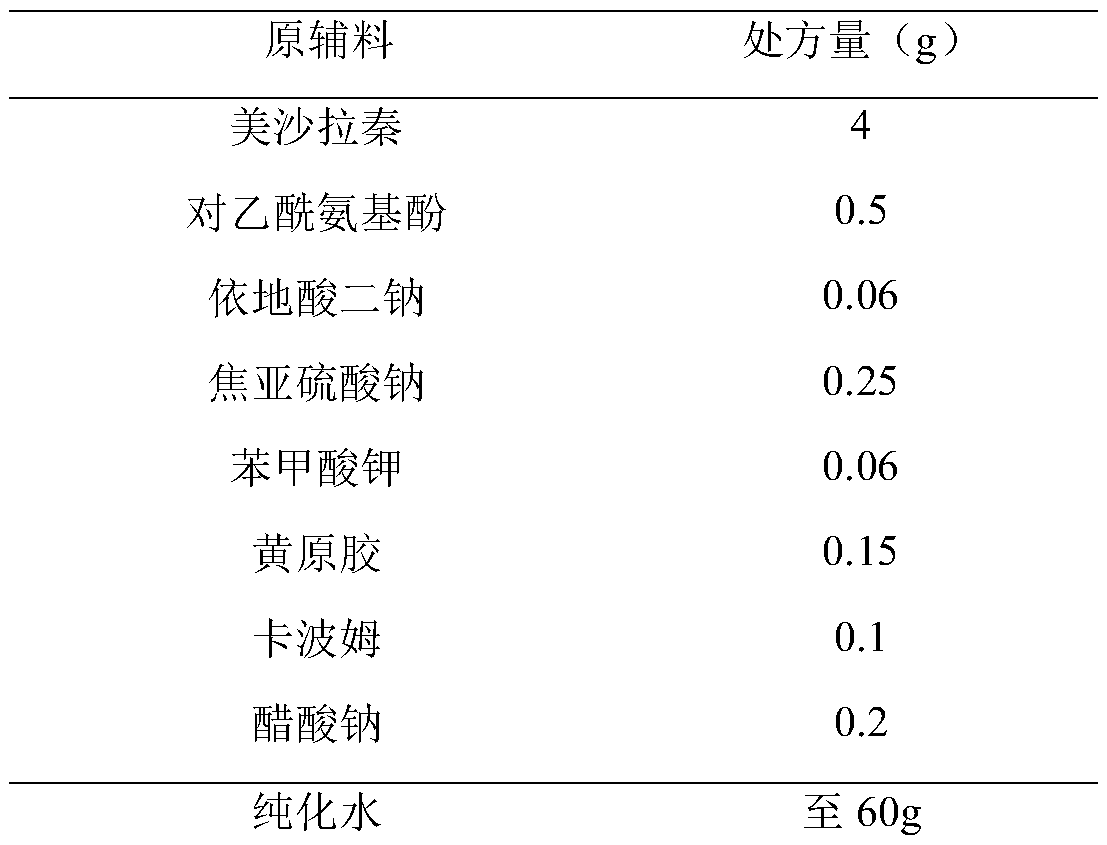
Embodiment 2
[0032]
[0033] Preparation Process:
[0034] (1) Get about 45g of new boiling water, add disodium edetate, potassium benzoate and potassium sodium acetate of recipe quantity successively, stir until dissolved.
[0035] (2) Slowly add the xanthan gum and carbomer of the recipe amount under stirring, and stir to swell.
[0036] (3) Add sodium metabisulfite, mesalazine and paracetamol in the prescribed amounts, stir and mix well.
[0037] (4) Homogenize, add new boiling water to the full amount, stir evenly, and fill.
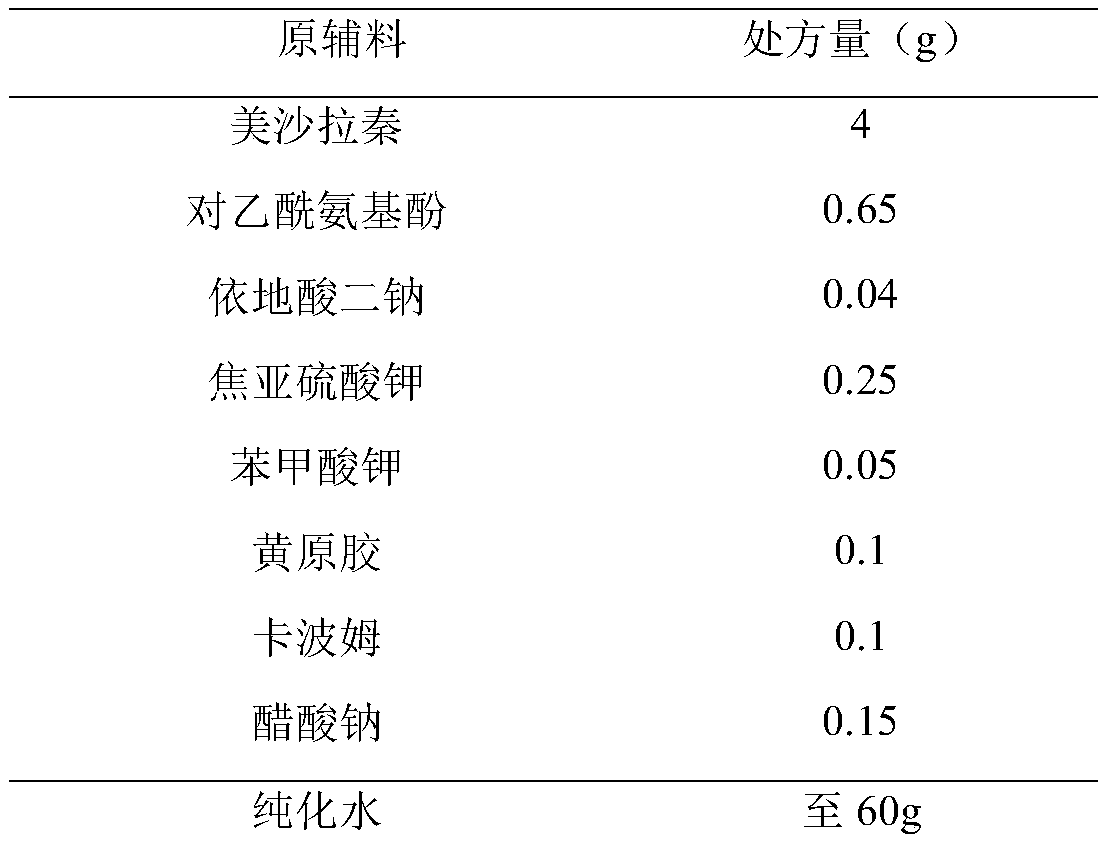
Embodiment 3
[0039]
[0040] Preparation Process:
[0041] (1) Get about 45g of new boiling water, add disodium edetate, potassium benzoate and potassium sodium acetate of recipe quantity successively, stir until dissolved.
[0042] (2) Slowly add the xanthan gum and carbomer of the recipe amount under stirring, and stir to swell.
[0043] (3) Add potassium metabisulfite, mesalazine and paracetamol in the prescribed amounts, stir and mix well.
[0044] (4) Homogenize, add new boiling water to the full amount, stir evenly, and fill.
[0045] Example 2
[0046] 1. Purpose of the test
[0047] Through the test, the improvement of symptoms of mice after using the medicine of the present invention and the medicine of the control group was observed.
[0048] 2. Test materials
[0049] 1. Tested drugs: medicine of the present invention, mesalazine enema solution (registration number H20150127), mesalazine suppository (national medicine approved character H20065650), mesalazine enteric-coa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com