Preparation method of palladium nano-catalyst for DFAFC (direct formic acid fuel cell)
A technology of formic acid fuel cell and palladium nanometer, applied in nanotechnology for materials and surface science, battery electrodes, nanotechnology, etc., can solve the problems of difficult realization of catalyst and commercial application, increased cost of catalyst, complicated process, etc. Achieve good catalytic activity and stability, improve Pd utilization, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
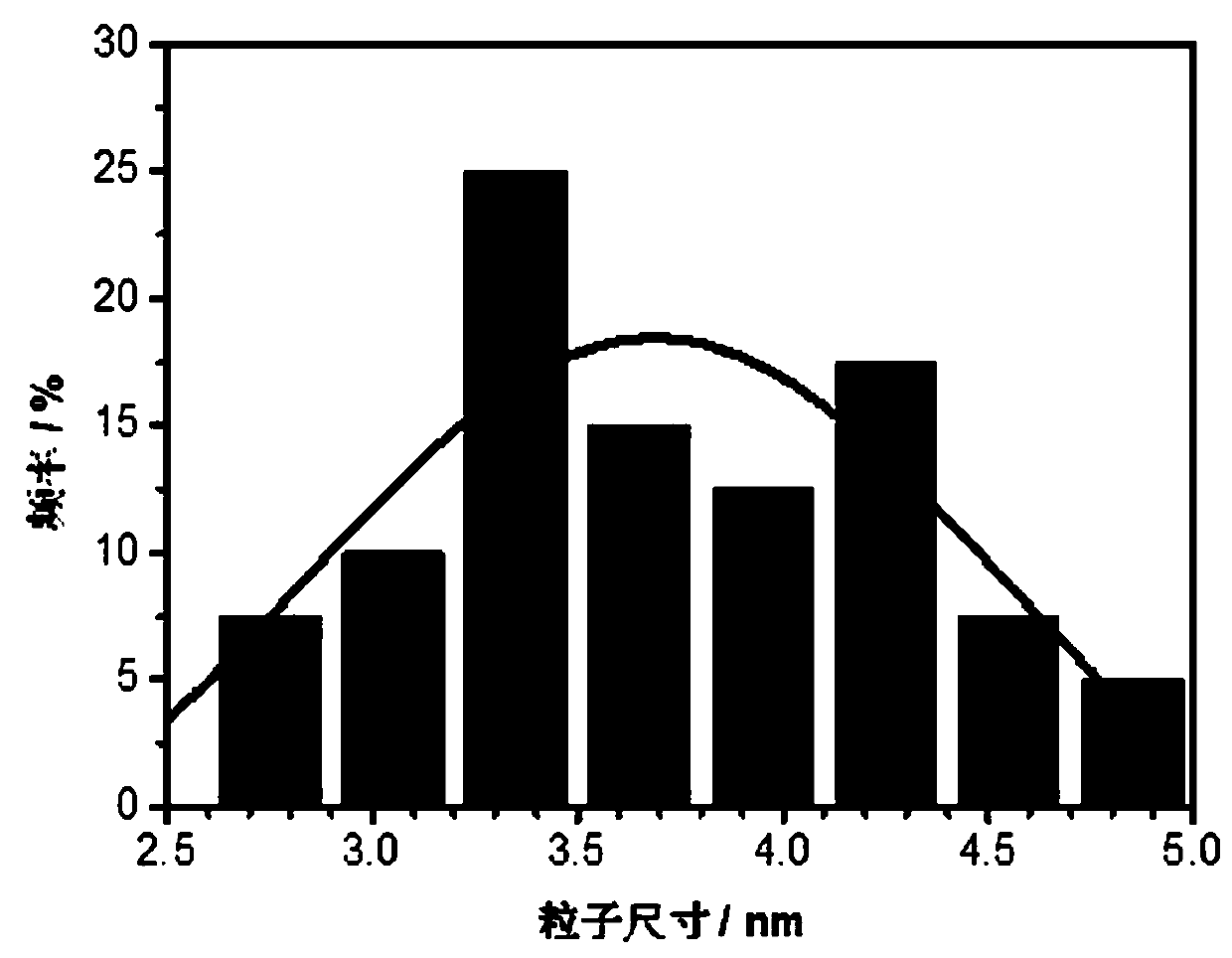
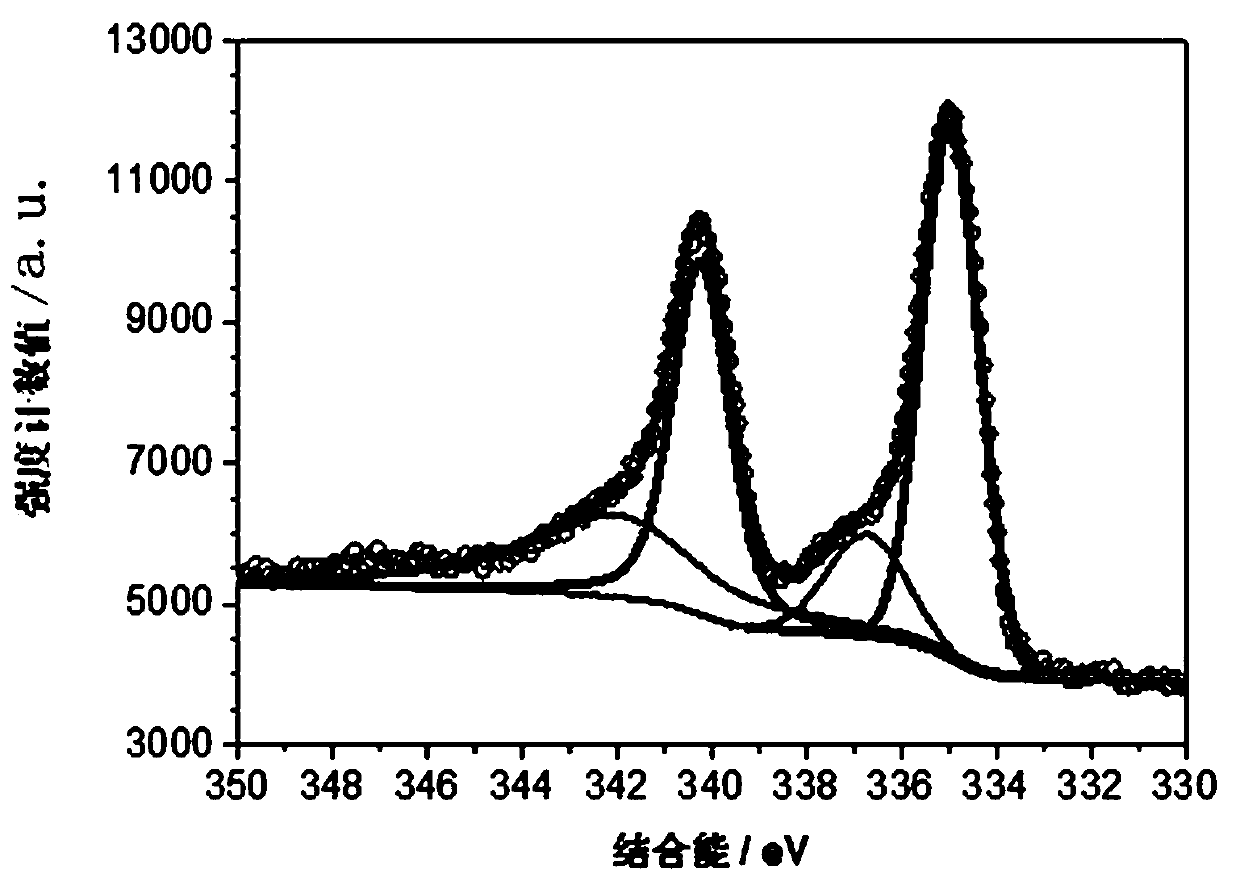
Image
Examples
Embodiment 1
[0032] 1) Add 40 mg of activated carbon to 20 mL of ethylene glycol, and disperse with ultrasonic and magnetic stirring for 15 min to obtain a suspension of activated carbon;
[0033] 2) Under ultrasonic and magnetic stirring, PdCl containing 10 mg Pd 2 The solution is added to the suspension, and soaked for 1-2 hours to obtain an impregnation solution;
[0034] 3) adjusting the pH of the impregnation solution to 10.5 with NaOH aqueous solution to obtain a colloidal solution;
[0035] 4) Stir the colloidal solution for 2 hours, then pass in argon to exhaust oxygen for 30 minutes;
[0036] 5) Dissolve 178mg of sodium borohydride in 10mL of 0.001M NaOH aqueous solution as a reducing agent, quickly and evenly add the reducing agent into the colloidal solution, and continue stirring for about 2 hours to completely reduce the palladium metal precursor;
[0037] 6) After filtering and washing, the obtained product was vacuum-dried at 80° C. for 10 h, and ground to obtain a palladi...
Embodiment 2
[0044] Preparation of high-dose Pd / C catalyst:
[0045] The steps in Example 1 were enlarged proportionally, 400 mg of activated carbon was added to 200 mL of ethylene glycol, and ultrasonically and magnetically stirred to disperse for 15 min to obtain a suspension of activated carbon; under ultrasonically and magnetically stirred, the PdCl 2 The solution is added to the suspension, and soaked for 2-4 hours to obtain an impregnation liquid; the pH of the impregnation liquid is adjusted to 10.5 with NaOH aqueous solution to obtain a colloidal liquid; after the colloidal liquid is stirred for 2 hours, argon gas is introduced to exhaust oxygen 30min; 1780mg of sodium borohydride was dissolved in 100mL of 0.001M NaOH aqueous solution as a reducing agent, and the reducing agent was added into the colloid liquid by means of multi-point addition, and the stirring reaction was continued for about 3 hours to completely reduce the palladium metal precursor; then filtered, After washing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com