Oxygen storage solid solution loaded cobalt-based catalyst for hydrogen production by autothermal reforming of acetic acid
A technology of cobalt-based catalyst and autothermal reforming, applied in hydrogen, inorganic chemistry, chemical instruments and methods, etc., can solve the problems of easy oxidation of active components, reduced catalyst activity, poor selectivity, etc., and achieve good oxygen storage capacity, Effects of inhibiting sintering and increasing fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0027] Weigh 2.117g of Co(NO 3 ) 2 ·6H 2 O, 6.918g of Ce(NO 3 ) 3 ·6H 2 O, 1.419g of ZrO(NO 3 ) 2 2H 2 O, add 50mL of deionized water to make solution #1; weigh 11.139g of NaOH, add 200mL of deionized water to make solution #2; #1 and solution #2 were added dropwise into a beaker and kept stirring for co-precipitation reaction for 1 hour, and continued to stir and age for 18 hours; after the aging was completed, the mixture was suction filtered and washed 3 times, and the obtained precipitate was dried in a drying oven at 105°C for 18 hours to obtain Catalyst precursor; the precipitate was calcined at 700°C for 4 hours to obtain CDUT-CCZ-31 catalyst. The molar composition of the catalyst is (CoO 4 / 3 ) 1.46 (CeO 2 ) 3.00 (ZrO 2 ) 1.00 ; According to the weight percentage, the composition is: 15.1% of cobalt tetroxide, 67.9% of cerium dioxide, and 17.0% of zirconium dioxide.
[0028] The reactivity evaluation of autothermal reforming of acetic acid was carried out...
Embodiment 1
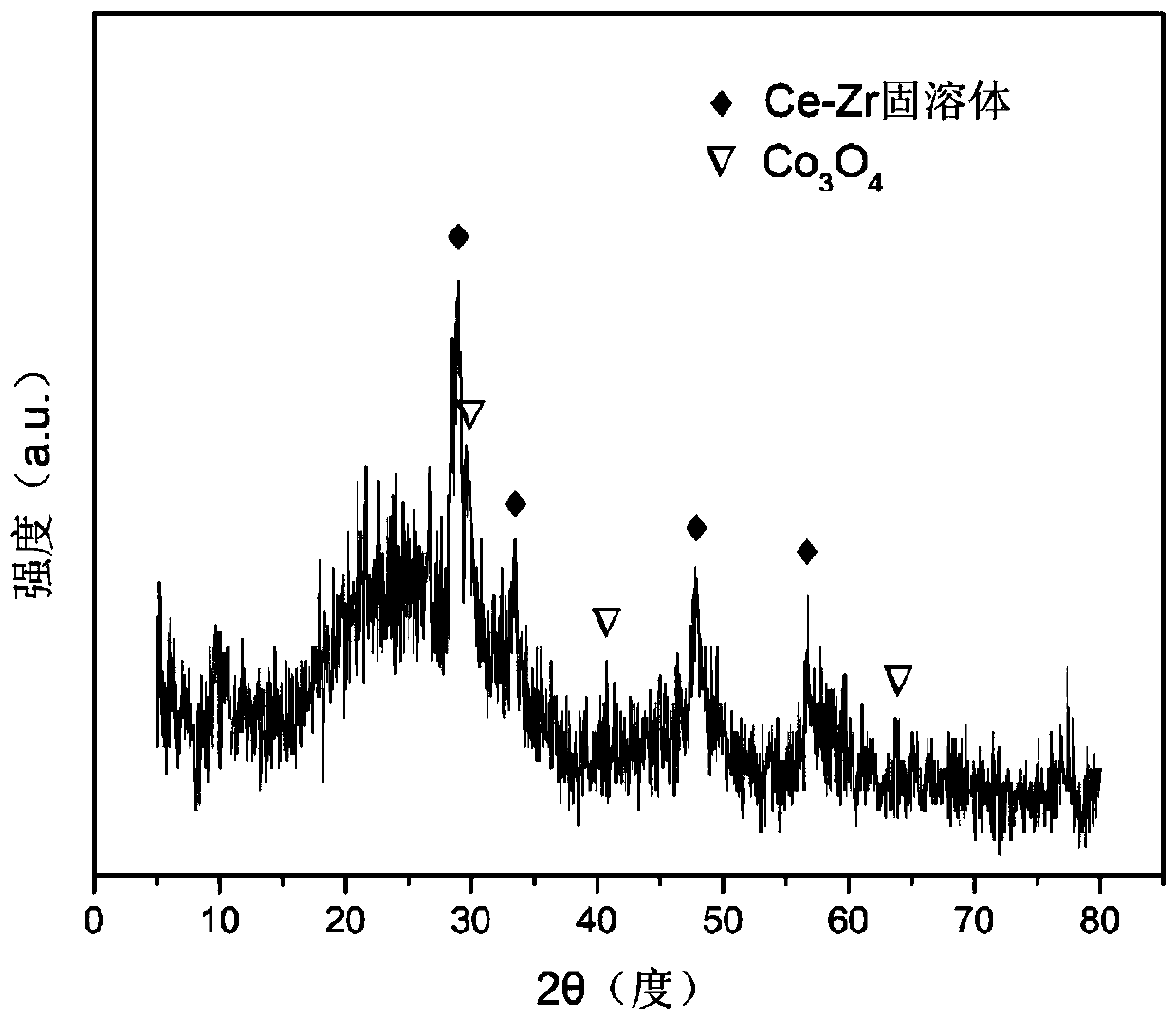
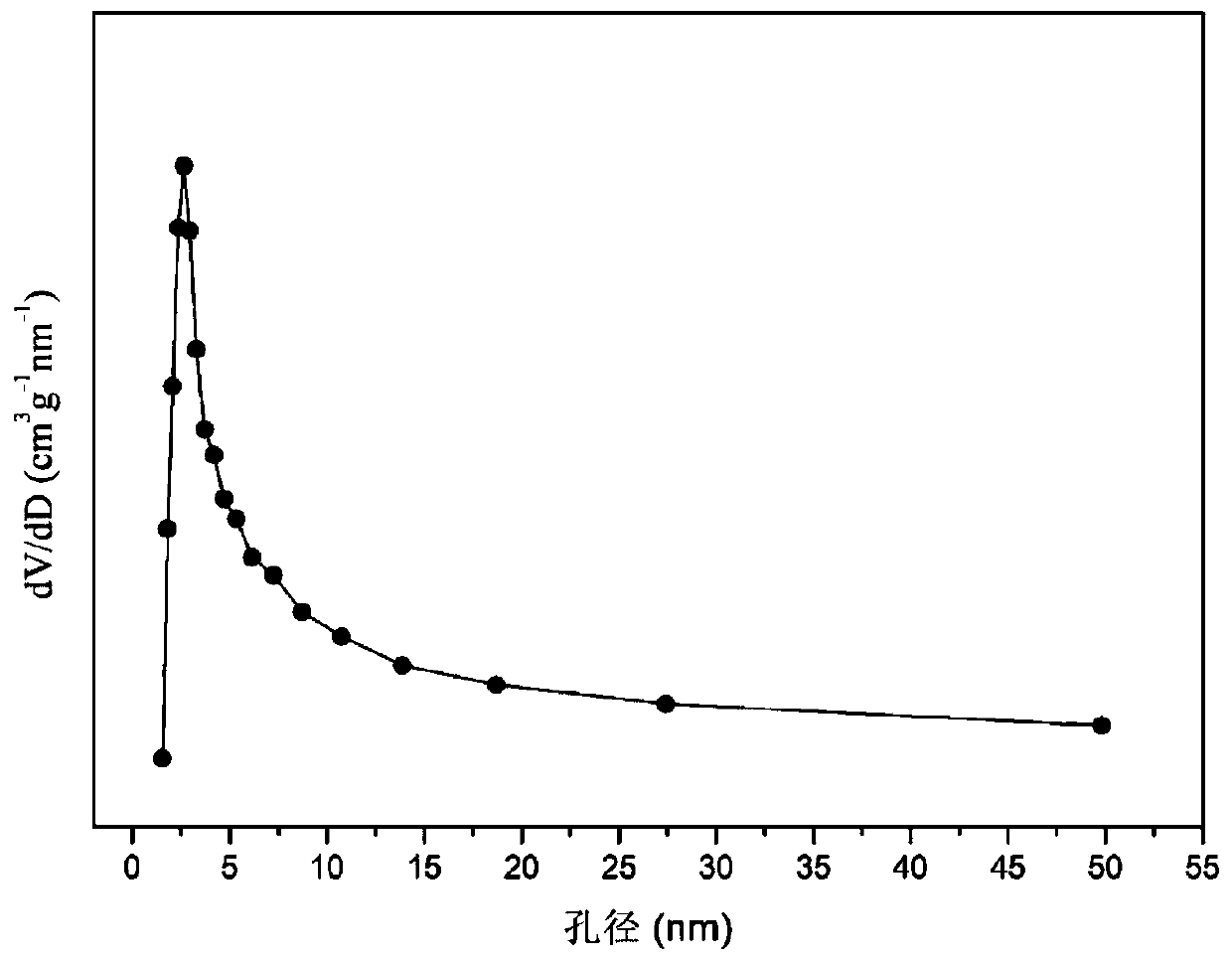
[0031] Weigh 2.110g of Co(NO 3 ) 2 ·6H 2 O, 4.997g of Ce(NO 3 ) 3 ·6H 2 O, 3.076g of ZrO(NO 3 ) 2 2H 2 O, add 50mL of deionized water to prepare solution #1; weigh 9.003g of NaOH, add 150mL of deionized water to prepare solution #2; follow-up steps are the same as reference example 1, and the precipitate is roasted at 700°C for 4 hours Finally, the CDUT-CCZ-11 catalyst was obtained, whose main components were oxygen storage solid solution (Ce-Zr-Ox) and highly dispersed Co 3 o 4 , the typical structure is shown in the X-ray diffraction pattern (attached figure 1 ), it also has a mesoporous structure, and the typical structure is shown in the attached figure 2 shown. The molar composition of the catalyst is (CoO 4 / 3 ) 0.68 (CeO 2 )(ZrO 2 ), according to the weight percent composition: tricobalt tetroxide is 15.1%, ceria is 48.5%, zirconium dioxide is 36.4%.
[0032] The activity of the CDUT-CCZ-11 catalyst was investigated by the autothermal reforming reaction ...
Embodiment 2
[0034] Weigh 2.117g of Co(NO 3 ) 2 ·6H 2 O, 2.723g of Ce(NO 3 ) 3 ·6H 2 O, 5.027g of ZrO(NO 3 ) 2 2H 2 O, add 50mL of deionized water to prepare solution #1; weigh 6.501g of NaOH, add 165mL of deionized water to prepare solution #2; follow-up steps are the same as reference example 1, and the precipitate is roasted at 700°C for 4 After hours, the CDUT-CCZ-13 catalyst was obtained, whose main components were oxygen storage solid solution (Ce-Zr-Ox) and highly dispersed Co 3 o 4 , the typical structure is shown in the X-ray diffraction pattern (attached figure 1 ), it also has a mesoporous structure, and the typical structure is shown in the attached figure 2 shown. The molar composition of the catalyst is (CoO 4 / 3 ) 0.15 (CeO 2 ) 1.00 (ZrO 2 ) 3.00 , according to the weight percent, the composition is: 15.0% of cobalt tetroxide, 26.1% of cerium dioxide, and 58.9% of zirconium dioxide.
[0035] The activity of the CDUT-CCZ-13 catalyst was investigated by the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com