Homogeneous oxidation synthesis method of isosulfan blue and the isosulfan blue synthesized by the method
A synthesis method and homogeneous oxidation technology, applied in the field of isothiocyanate, can solve the problems of low purity of reaction solution, large environmental pollution, difficulty in product purification and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
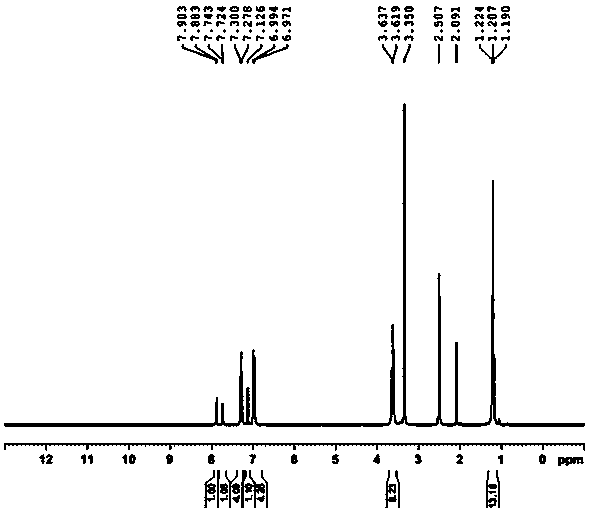
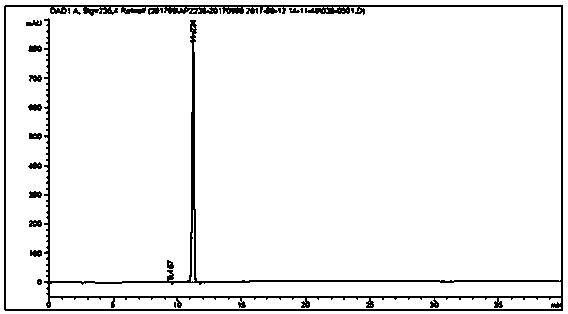
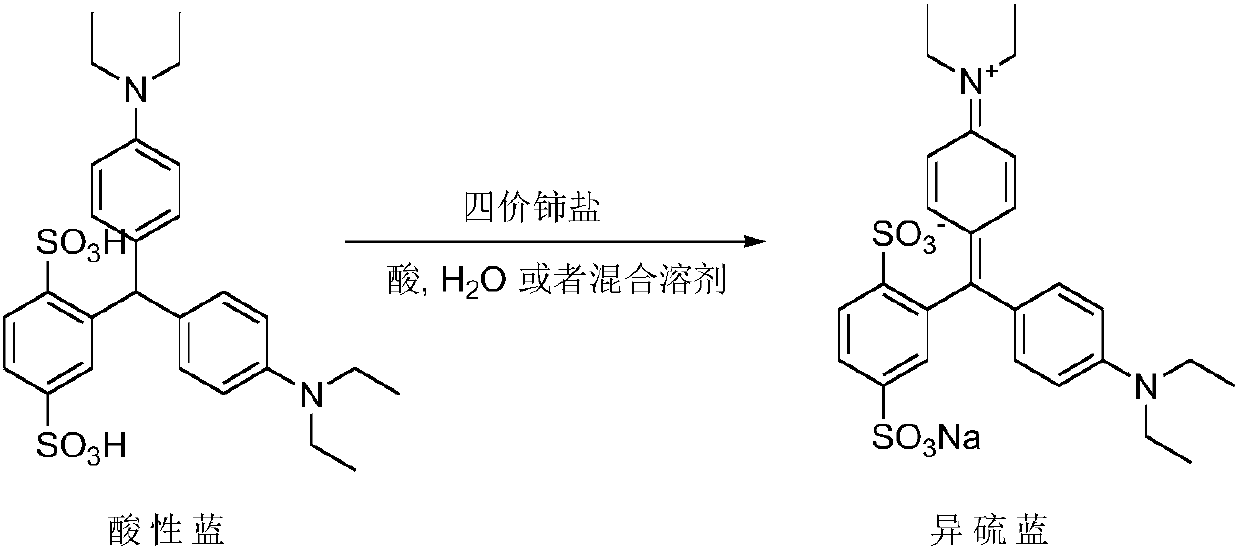
[0039] Add acid blue (10.0g, 18.0mmol), ceric ammonium nitrate (30.0g, 54.0mmol), water (400ml), concentrated nitric acid (2.2g, 22.5mmol), react at -10~10℃ for 3 hours, the central control shows that the raw material is less than 0.5%, and the deethylated impurity is 1.0%; adjust the pH to 7~10 with 20% sodium carbonate solution, filter, and depressurize the filtrate Concentrate; add 100ml of methanol to the residue, filter to remove inorganic salts; concentrate the filtrate under reduced pressure, dissolve the residue in 15ml of water, adjust the pH to acidic with acid, stir for 30min, and filter to obtain isothiocyanic acid; isothiocyanic acid is dissolved in 25ml of water and 20% sodium carbonate solution to adjust the pH to 7-10; add acetone (500ml) to the obtained solution, stir for 1h; filter, wash the filter cake with acetone (150ml) twice, and dry under vacuum at 40°C for 8h to obtain 4.1g of isosulfur blue , purity 99.6%, deethylation impurity 0.12%, unknown single i...
Embodiment 2
[0044] Add acid blue (10.0g, 18.0mmol), ceric ammonium nitrate (30.0g, 54.0mmol), water (400ml), sulfuric acid ( 2.2g, 22.5mmol), react at -10~10°C for 8 hours, the central control shows that the raw material is less than 2.0%, and the deethylated impurity is 0.3%; 20% sodium carbonate solution is adjusted to pH 7~10, filtered, and the filtrate is concentrated under reduced pressure; Add 100ml of methanol to the residue, filter to remove inorganic salts, and concentrate the filtrate under reduced pressure; dissolve the residue in 15ml of water, adjust the pH to acidic with acid, stir for 30min, and filter to obtain isothiocyanic acid; dissolve isothiocyanic acid in 25ml of water , 20% sodium carbonate solution to adjust the pH to 7-10; the resulting solution was added with acetone (500ml), stirred for 1h; filtered, the filter cake was washed twice with acetone (150ml), and vacuum-dried at 40°C for 8h to obtain 3.9g of isosulfur blue, the purity 99.7%, deethylation impurity 0.0...
Embodiment 3
[0046]Add acid blue (10.0g, 18.0mmol), ammonium cerium sulfate (32.0g, 54.0mmol), water (400ml), sulfuric acid ( 2.2g, 22.5mmol), react at -10~10°C for 8 hours, the central control shows that the raw material is less than 0.5%, and the deethylated impurity is 0.3%; 20% sodium carbonate solution is adjusted to pH 7~10, filtered, and the filtrate is concentrated under reduced pressure; Add 100ml of methanol to the residue, filter to remove inorganic salts, and concentrate the filtrate under reduced pressure; dissolve the residue in 15ml of water, adjust the pH to acidic with acid, stir for 30min, and filter to obtain isothiocyanic acid; dissolve isothiocyanic acid in 25ml of water , 20% sodium carbonate solution to adjust the pH to 7-10; the resulting solution was added with acetone (500ml), stirred for 1h; filtered, the filter cake was washed twice with acetone (150ml), and vacuum-dried at 40°C for 8h to obtain 3.9g of isosulfur blue, the purity 99.7%, deethylation impurity 0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com