Application of microorganism in treatment and/or prevention of immune-mediated intestinal diseases
An intestinal disease, immune-mediated technology, applied to products containing Bifidobacterium breve, which can address the therapeutic role in the treatment and/or prevention of intestinal diseases induced by immune checkpoint blockade no clear questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1 confirms functional bacterial strain
[0072] 1. Preparation of colitis model mice induced by CTLA-4 blockade
[0073] Give mice vancomycin (0.5g / L, Sigma) to change intestinal commensal bacteria, and after at least 14 days, add 2-4% heparin-like sulfated polysaccharide (DSS, MP Biomedicine) into the drinking water of mice and take 7 -12 days. At the beginning of DSS treatment, mice were injected with 200ug anti-CTLA-4 mAb.
[0074] 2. Test plan
[0075] 1) Body weight change test
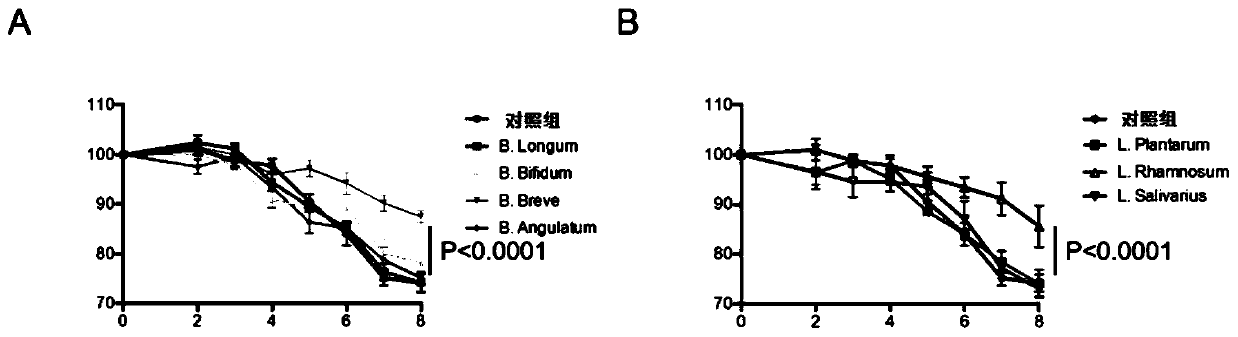
[0076] The colitis model mice were inoculated with four types of bifidobacteria: Bifidobacterium breve (B.Breve), Bifidobacterium longum (B.Longum), Bifidobacterium bifidum (Bifidum), Bifidobacterium lactis ( B.Angulatum), each mouse was orally fed with 1x10 9 CFU, the control group was given the same amount of PBS, and then the body weight was monitored every day.
[0077] Inoculate colitis model mice with three kinds of lactic acid bacteria: Lactobacillus plantarum (L.Pl...
Embodiment 2
[0086] Example 2 Effects of Bifidobacteria on the composition of intestinal flora and the abundance of intestinal bacteria
[0087] Bifidobacterium breve modulates CTLA-4 blockade-induced intestinal disease in relation to Treg cells. This assay is expected to verify whether Treg cells directly regulate the microbial composition and gut microbiota composition produced by Bifidobacterium breve in individuals with Treg cells and Influence of intestinal bacterial abundance.
[0088] 1. Mice and groups:
[0089] The mice used in the experiment were 6-14-week-old female mice, which were raised in a specific pathogen-free facility of Shanghai Jiaotong University. Mouse experiments were approved by the Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine.
[0090] Take 50 C57BL / 6N mice and randomly divide them into five groups, 10 mice in each group. One DTR is carried on the Treg cells of two groups of mice to instantly deplete Treg cells (De-Treg), and...
Embodiment 3
[0100] Example 3 Effect of Bifidobacterium breve on the expression level of inflammation-related genes
[0101] 1. Bifidobacterium breve inhibits the proliferation of Treg cells
[0102] The effector T cells (Teff) were co-cultured with the Treg cells treated with Bifidobacterium breve, and the effector T cells were co-cultured with the Treg cells treated with PBS, and the proliferation ratio was compared. The results were as follows: Image 6 As shown, the proliferative ability of Treg cells decreased significantly after being treated with Bifidobacterium breve.
[0103] 2. Deletion of the IL10 gene and / or blocking the expression of IL-22 can cause intestinal diseases
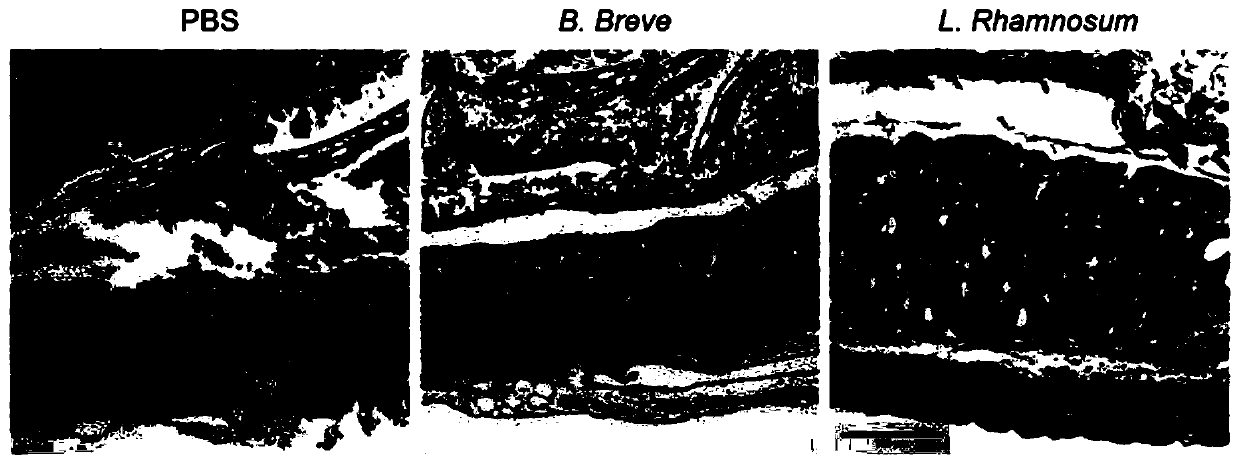
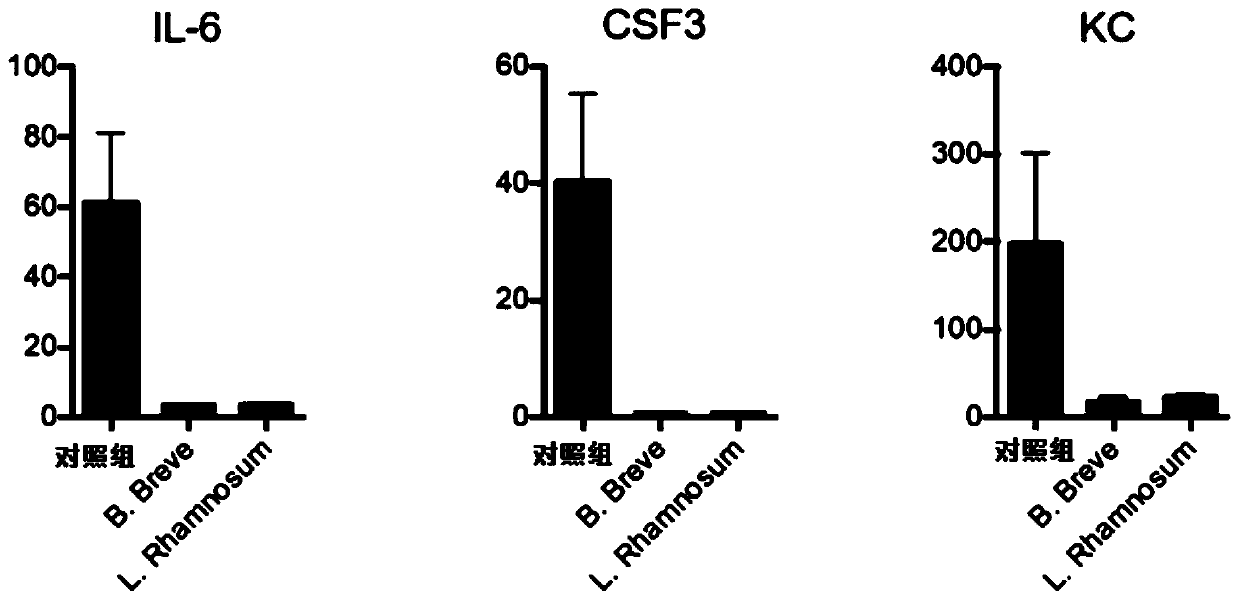
[0104] IL10 knockout mice, IL-22 antibody-injected and IgG-treated mice (control group) were prepared according to the steps in Example 1 to prepare CTLA-4 blocking-induced colitis model mice, and then the three kinds of mice were treated with Administration of Bifidobacterium breve. The body weight change,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com