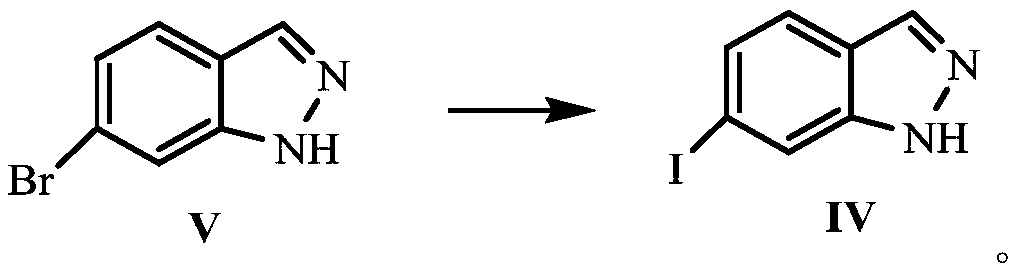
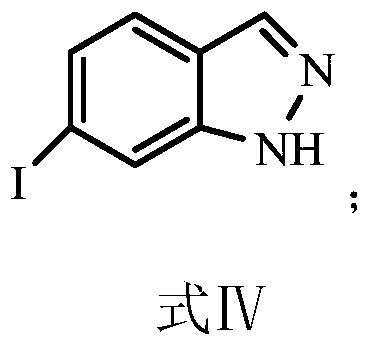
Synthesis method of 6-iodine-1H-indazole
A synthetic method and indazole technology, applied in the field of synthesis of 6-iodo-1H-indazole, can solve the problems of large pollution, long steps, low yield of synthetic route, etc., and achieve low impurity content, stable process and smooth operation flow simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A kind of synthetic method of 6-iodo-1H-indazole, synthetic route is as follows:
[0029]
[0030] The synthetic method of above-mentioned 6-iodo-1H-indazole comprises the following steps:
[0031] Weigh 5.0g of the compound of formula V and 13.38g of potassium iodide in the reactor, add 50mL of 1,4-dioxane, and then add 0.2g of tetrabutylammonium iodide, based on the compound of formula V, 0.51g Cuprous iodide and 0.47g N,N-dimethylethylenediamine (0.2equiv.) were added to the reactor, the temperature was raised to reflux, and the reflux reaction was carried out for 48h. After the reaction was completed, the reaction solution was filtered after cooling down to room temperature. , Wash the filter cake 3 times with 4-dioxane, combine the filtrates, concentrate the obtained filtrate, dissolve the concentrate in ethyl acetate, wash the ethyl acetate layer with 13% ammonia solution, separate the organic phase, concentrate the organic phase, and concentrate After recryst...
Embodiment 2
[0033] A kind of synthetic method of 6-iodo-1H-indazole, synthetic route is as follows:
[0034]
[0035] The synthetic method of above-mentioned 6-iodo-1H-indazole comprises the following steps:
[0036] Weigh 4.5g of the formula V compound and 12.05g of potassium iodide in the reactor, add 45mL of 1,4-dioxane, add 0.22g of tetrabutylammonium iodide, in terms of the formula V compound, 0.3equiv The cuprous iodide and N,N-dimethylethylenediamine were added to the reactor, the temperature was raised to reflux, and the reflux reaction was carried out for 54 hours. Wash the filter cake 3 times with six rings, combine the filtrate, concentrate the obtained filtrate, dissolve the concentrate with ethyl acetate, wash the ethyl acetate layer with 20% ammonia solution, separate the organic phase, concentrate the organic phase, and reconcentrate the concentrate with acetonitrile After crystallization, the compound of formula IV, 6-iodo-1H-indazole, was obtained with a yield of 84.2...
Embodiment 3
[0038] A kind of synthetic method of 6-iodo-1H-indazole, synthetic route is as follows:
[0039]
[0040] The synthetic method of above-mentioned 6-iodo-1H-indazole comprises the following steps:
[0041] Weigh 5.5g of formula V compound and 14.71g of potassium iodide in the reactor, add 55mL of 1,4-dioxane, add 0.18g of tetrabutylammonium iodide, in terms of formula V compound, 0.1equiv The cuprous trifluoromethanesulfonate and N,N-dimethylethylenediamine were added to the reactor, the temperature was raised to reflux, and the reflux reaction was carried out for 46 hours. After the reaction was completed, after cooling down to room temperature, the reaction liquid was filtered, and the obtained filtrate was concentrated. After the concentrate was dissolved in ethyl acetate, the ethyl acetate layer was washed with 10% ammonia solution, the organic phase was separated, the organic phase was concentrated, and the concentrate was recrystallized from acetonitrile to obtain the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com