Preparation method of iron-catalyzed phosphazene compound
A technology of phosphazene and compounds, which is applied in the field of preparation of phosphazene compounds, can solve the problems of low total yield, small application range of substrates, unstable raw materials and intermediates, etc. Mild conditions and good substrate compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
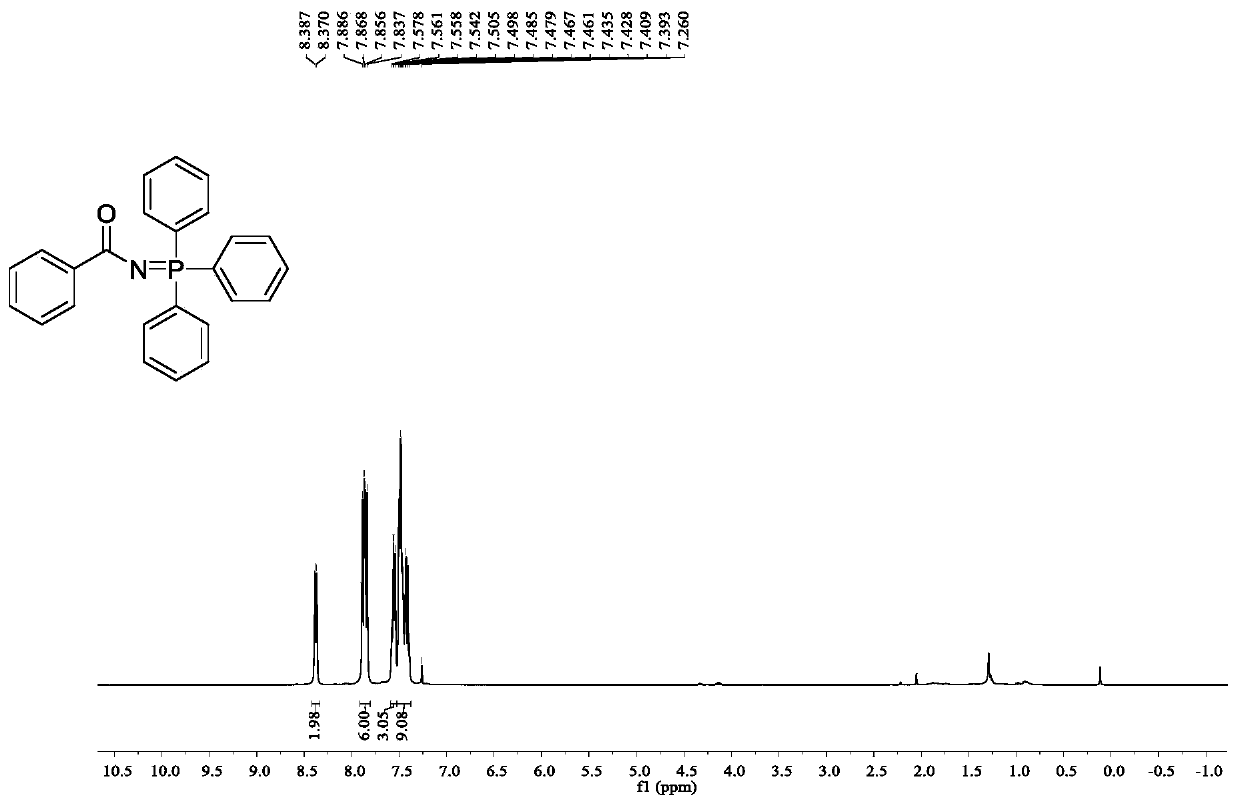
[0028] Embodiment 1: N-(triphenyl-λ 5 -Synthesis of phosphanylidene)benzamide
[0029] Accurately weigh 3-phenyl-1,4,2-dioxazol-5-one (48.9mg, 0.3mmol), triphenylphosphine (157.2mg, 0.6mmol), ferrous chloride (1.9mg, 5mol %) into a 25mL Schlenk reaction flask, and then added toluene (2mL), placed under light conditions (1W, 280nm) and reacted at 10°C for 36h. After the reaction, the solvent was removed under reduced pressure, and petroleum ether / ethyl acetate was used as eluent, and the product was separated on a silica gel column, and the yield of the product was 95%.
[0030]
[0031] 1 H NMR (400MHz, CDCl 3 )δ8.41-8.35(m,2H),7.91-7.81(m,6H),7.59-7.53(m,3H),7.52-7.38(m,9H).
Embodiment 2
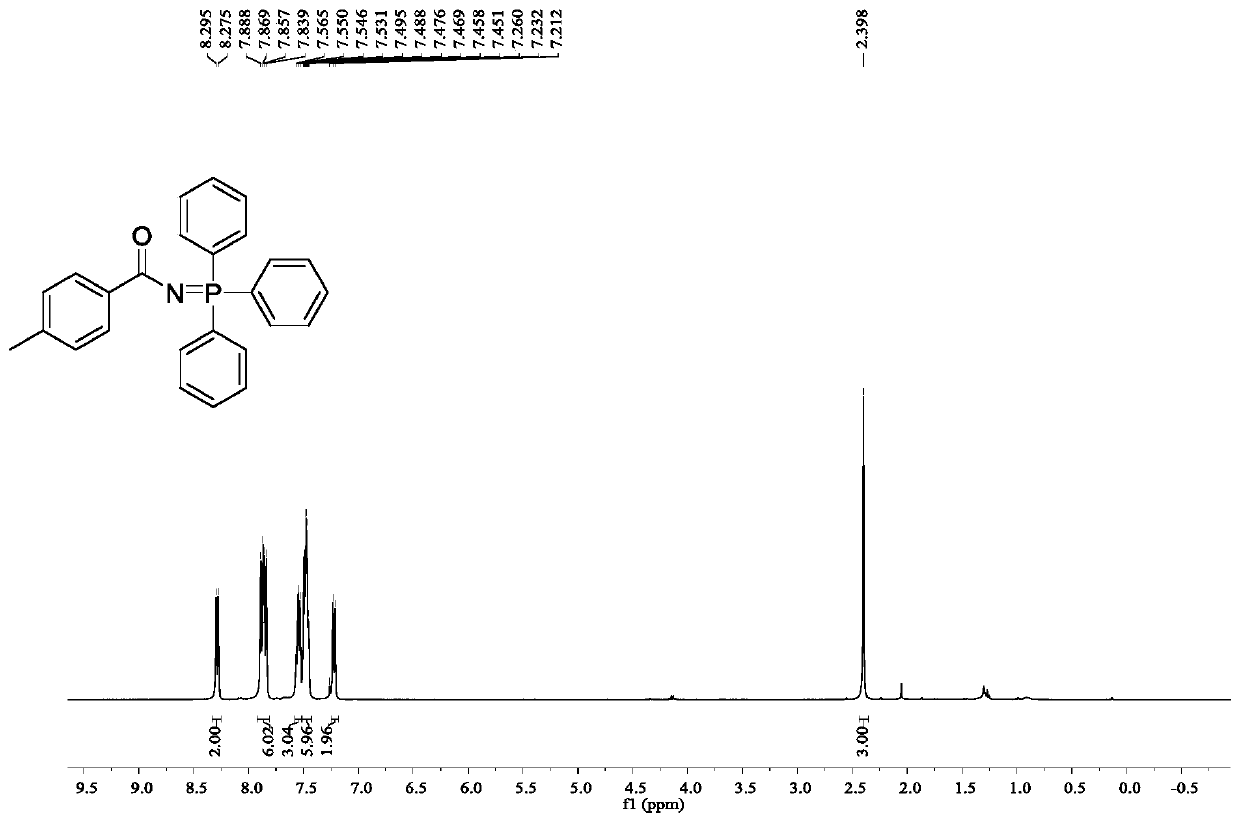
[0032] Embodiment 2: N-(triphenyl-λ 5 -Synthesis of 4-methylbenzamide
[0033] Accurately weigh 3-(4-methylphenyl)-1,4,2-dioxazol-5-one (53.1mg, 0.3mmol), triphenylphosphine (78.6mg, 0.3mmol), dinonylcarbonyl Iron (1.1 mg, 1 mol%) was added to a 25 mL Schlenk reaction flask, then dichloromethane (2 mL) was added, and the mixture was reacted at 20° C. for 24 h under light conditions (15 W, 420 nm). After the reaction was completed, the solvent was removed under reduced pressure, and petroleum ether / ethyl acetate was used as the eluent to separate on a silica gel column. The yield of the product was 91%.
[0034]
[0035] 1 H NMR (400MHz, CDCl 3 )δ8.28(d, J=8.0Hz, 2H), 7.91-7.82(m, 6H), 7.58-7.52(m, 3H), 7.51-7.43(m, 6H), 7.22(d, J=8.0Hz ,2H),2.40(s,3H).
Embodiment 3
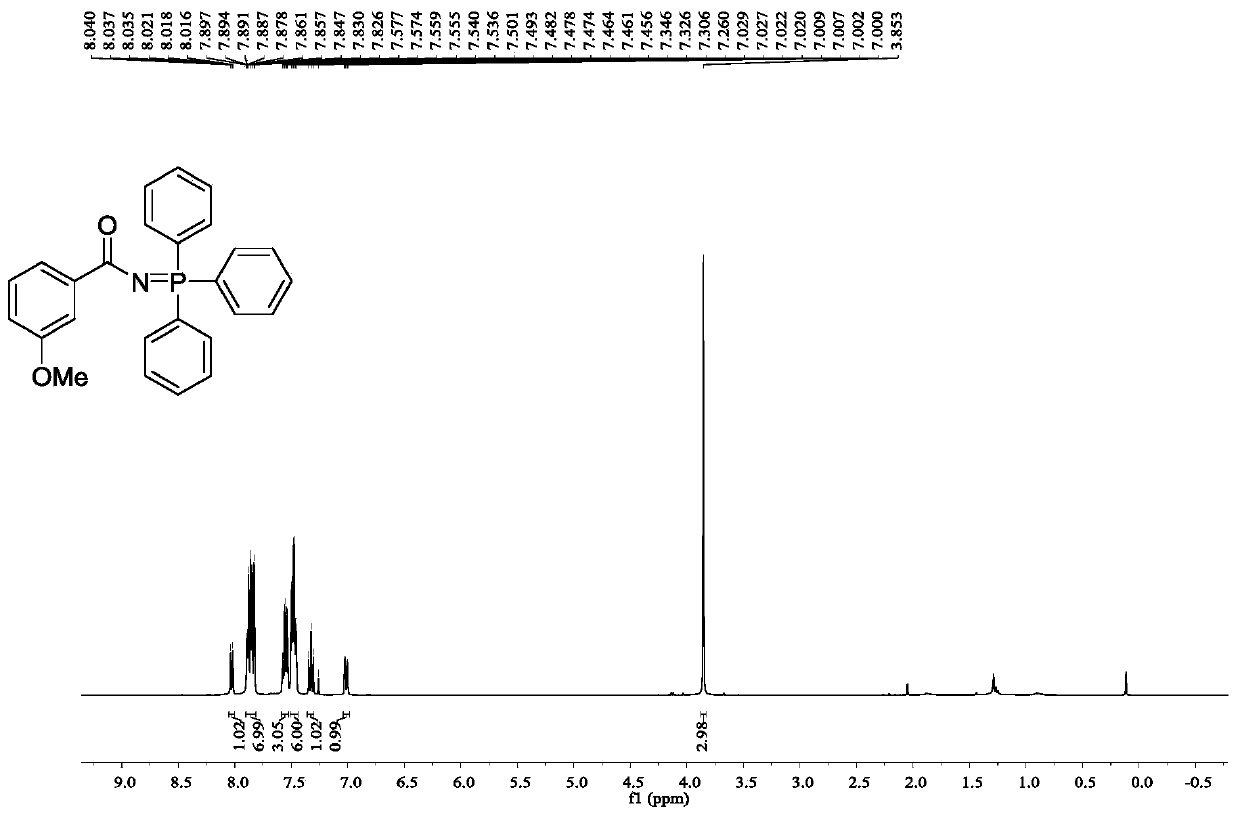
[0036] Embodiment 3: N-(triphenyl-λ 5 -Synthesis of 3-methoxybenzamide
[0037] Accurately weigh 3-(3-methoxyphenyl)-1,4,2-dioxazol-5-one (57.9mg, 0.3mmol), triphenylphosphine (157.2mg, 0.6mmol), chloride Iron (24.3mg, 50mol%) was added into a 50mL Schlenk reaction flask, and then toluene (30mL) was added, and the mixture was reacted at 20°C for 8h under light conditions (25W, 450nm). After the reaction, the solvent was removed under reduced pressure, and petroleum ether / ethyl acetate was used as eluent, and the product was separated on a silica gel column, and the yield of the product was 90%.
[0038]
[0039] 1 H NMR (400MHz, CDCl 3 )δ8.05-8.00(m,1H),7.91-7.81(m,7H),7.59-7.53(m,3H),7.51-7.44(m,6H),7.36-7.30(m,1H),7.04- 6.98(m,1H),3.85(s,3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| power | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com