Sulfonated Lewis X trisaccharide and synthesis method and application thereof
A synthesis method and a sulfonation technology, which are applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of LeX and its derivatives being lengthy and complex, and no published literature has been found, and achieve high yield and synthesis. The effect of few steps and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0057] The embodiments of the present invention will be described in detail below. It should be noted that the embodiments are illustrative, not restrictive, and cannot limit the protection scope of the present invention.
[0058] The raw materials used in the present invention, unless otherwise specified, are conventional commercially available products; the methods used in the present invention, unless otherwise specified, are conventional methods in the art.
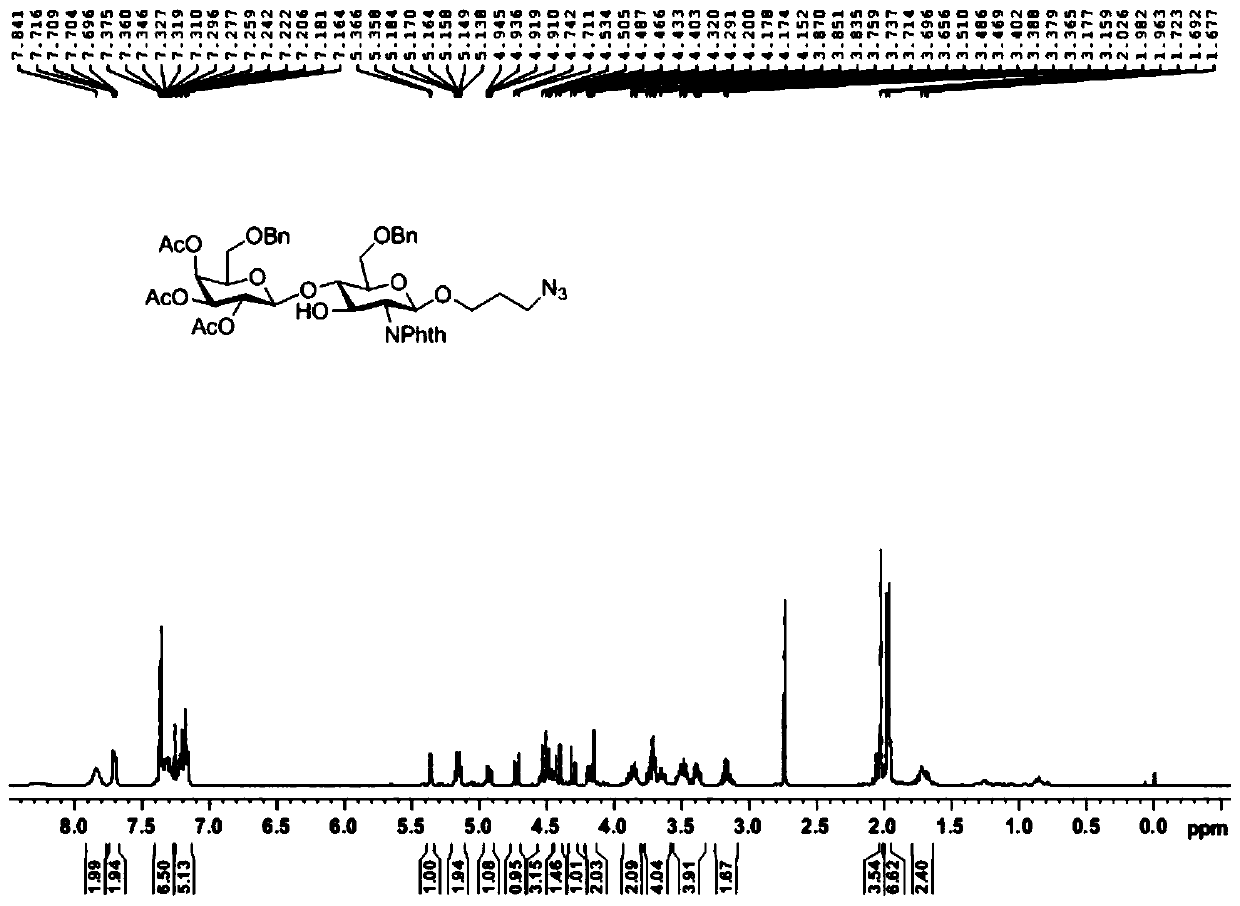
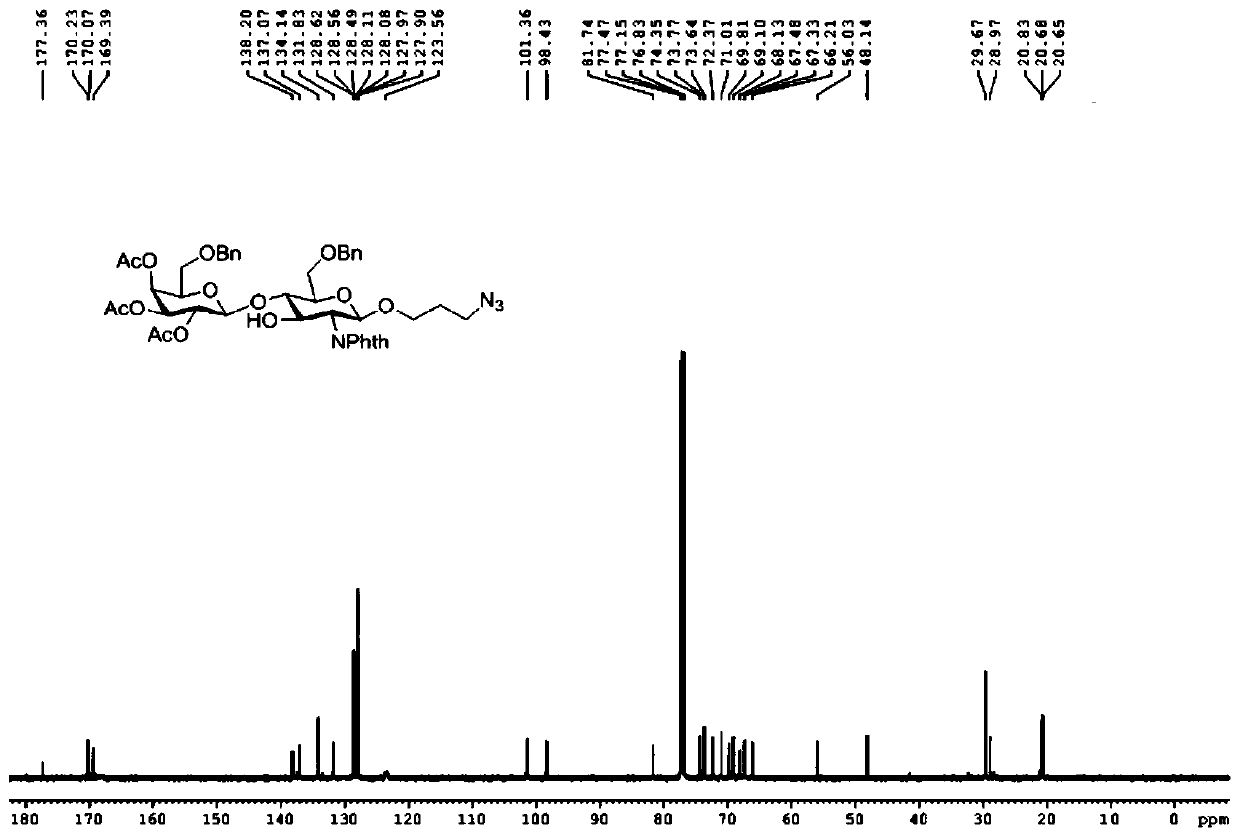
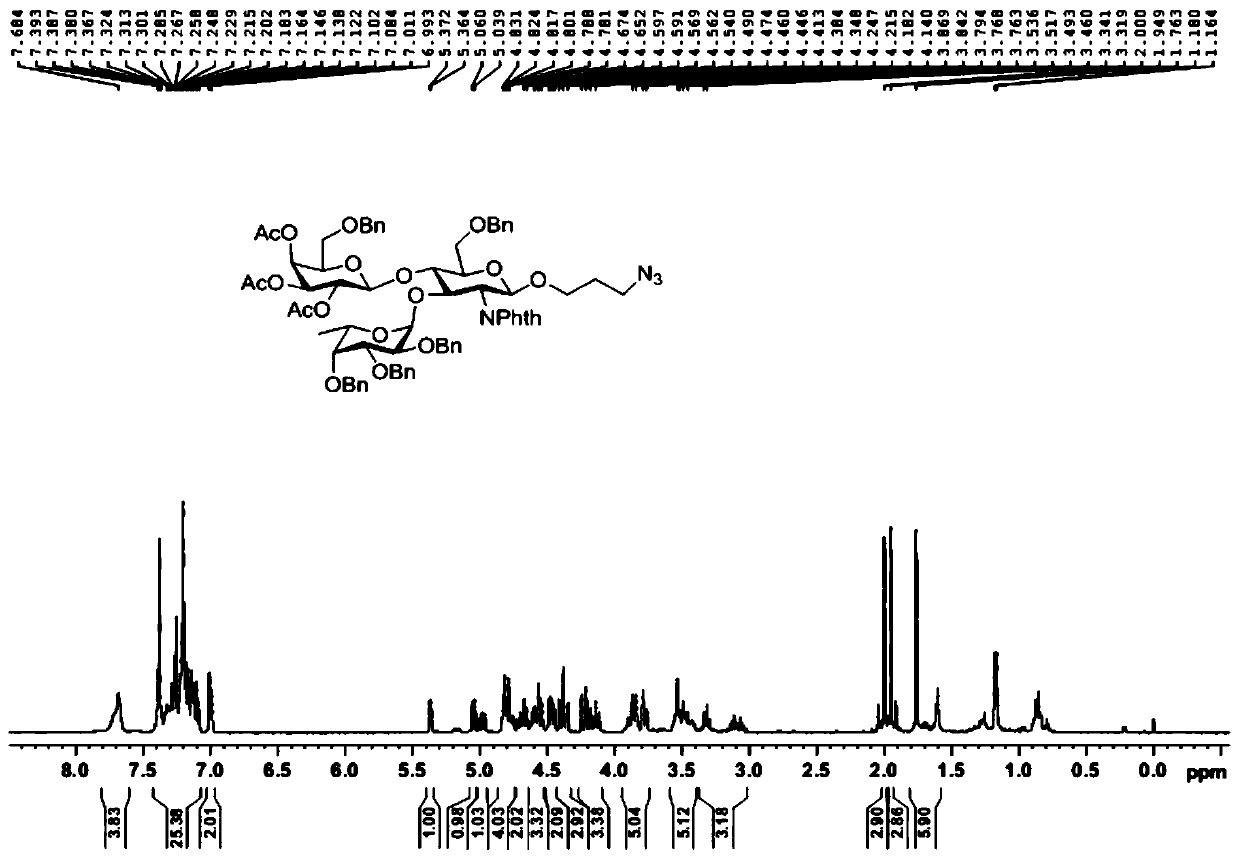
[0059] A kind of sulfonated Lewis X trisaccharide, its general structural formula is as follows:
[0060]
[0061] in:
[0062] R 1 , selected from hydrogen atom, α- or β-configuration serine residue, α- or β-configuration threonine residue, azide substituted alkyl, alkynyl substituted alkyl, mercapto substituted alkyl, α- or β-configuration substituted alkyl;
[0063] R 2 , selected from hydrogen atom, sulfonic acid group, phosphoric acid group, carbonic acid group.
[0064] A kind of synthetic method of sulf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com