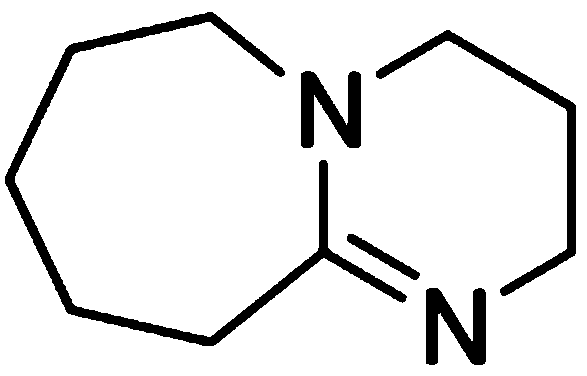
Method for synthesizing 1, 8-diazabicycle
A diazabicyclo and synthesis method technology, applied in the field of 1,8-diazabicyclo synthesis, capable of solving problems such as low yield and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] A kind of synthetic method of 1,8-diazabicyclo, with epsilon caprolactam as starting material, first react with hydroquinone in the presence of acrylonitrile, then pass dry Hydrogen chloride gas until the solution is saturated, continue to react under ice bath for more than 10 hours, suction filter under nitrogen protection, add benzyl chloride to react; mix the reaction product with sodium perborate, and then dissolve in dichloromethane in the presence of ethylenediaminetetraacetic acid Reflux for 8 hours; then add 3-tert-butyl salicylaldehyde, add 3-phenylpropionic acid at 45°C under stirring conditions, keep the temperature constant until the raw materials disappear completely, cool down to room temperature, add water to dilute 3 times, and then Add 4-nitrobenzyl chloride to stir the reaction, filter to obtain a solid, and then extract the filtrate with ethyl acetate three times, collect the solid phase, dry with anhydrous magnesium sulfate, and dry under reduced pres...
Embodiment 2
[0017] A kind of synthetic method of 1,8-diazabicyclo, with epsilon caprolactam as starting material, first react with hydroquinone in the presence of acrylonitrile, then pass dry Hydrogen chloride gas until the solution is saturated, continue to react under ice bath for more than 10 hours, suction filter under nitrogen protection, add benzyl chloride to react; mix the reaction product with sodium perborate, and then dissolve in dichloromethane in the presence of ethylenediaminetetraacetic acid Reflux for 8 hours; then add 3-tert-butyl salicylaldehyde, add 3-phenylpropionic acid at 45°C under stirring conditions, keep the temperature constant until the raw materials disappear completely, cool down to room temperature, add water to dilute 3 times, and then Add 4-nitrobenzyl chloride to stir the reaction, filter to obtain a solid, and then extract the filtrate with ethyl acetate three times, collect the solid phase, dry with anhydrous magnesium sulfate, and dry under reduced pres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com