Preparation method of organic phosphine flame retardant 1-hydroxyl phosphate
A technology of hydroxyphosphonite and flame retardant, which is applied in the field of 1-hydroxyalkylphosphonite, can solve the problems of hypophosphorous acid corrosion, limited application, explosion, etc., and achieve low cost, and the preparation method is environmentally friendly and safe Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Synthesis of sodium 1-hydroxybenzyl phosphinate
[0041] Add 1mol sodium hypophosphite and 1.5mol benzaldehyde into a 5L reaction kettle, add 2L DMF, plug a rubber stopper or a condenser, heat and react at 110°C for 24h, filter the reaction sample with suction after the reaction, wash and dry with alcohol, and the yield is 85%.
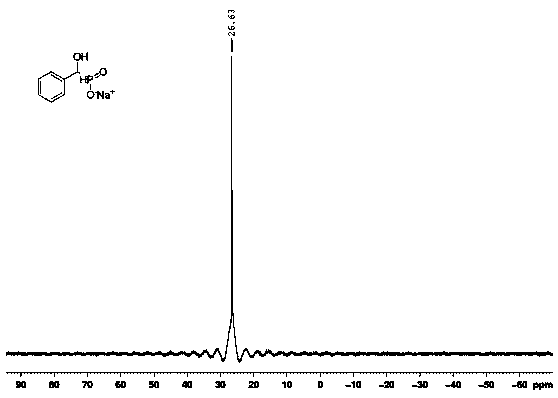
[0042] Sodium 1-Hydroxybenzylphosphinate, structural formula 1H NMR (400MHz, D2O): δ7.36-7.47(m, 5H), 7.50(dd, Jp-H=516.2Hz, JH-H=1.0Hz, 0.5H), 4.69-4.72(d, JH-H =9.0Hz, 1H); 13C NMR (100MHz, D2O): δ137.4, 128.6, 127.9, 127.1, 73.9 (d, JC-P = 105.9Hz), 31P NMR (162MHz, D2O): δ26.6. Phosphorus spectrum see figure 1 .
[0043] The synthesized product consists of the following:
[0044] Sodium 1-hydroxybenzylphosphinate: 97mol%;
[0045] Sodium bis(1-hydroxybenzyl)phosphonate: 2mol%
[0046] Sodium 1-hydroxybenzylphosphonate: 1mol%
Embodiment 2
[0047] Embodiment 2: Synthesis of sodium 1-hydroxybenzyl phosphinate
[0048] Add 1 mol of sodium hypophosphite and 1.75 mol of benzaldehyde into a 5L reaction kettle, add 2L of DMF, plug a rubber stopper or a condenser, heat and react at 110°C for 24 hours, filter the reaction sample after the reaction, wash and dry with alcohol, and the yield is 88%.
[0049] The synthesized product consists of the following:
[0050] Sodium 1-hydroxybenzylphosphonite: 98mol%;
[0051] Sodium bis(1-hydroxybenzyl)phosphonate: 1.5mol%;
[0052] Sodium 1-hydroxybenzylphosphonate: 0.5 mol%.
Embodiment 3
[0053] Embodiment 3: Synthesis of sodium 1-hydroxybenzyl phosphonate
[0054] Add 1mol sodium hypophosphite and 2mol benzaldehyde into a 5L reaction kettle, add 2L DMF, plug a rubber stopper or a condenser, heat and react at 110°C for 24h, filter the reaction sample after the reaction, wash and dry with alcohol, and the yield is 89%.
[0055] The synthesized product consists of the following:
[0056] Sodium 1-hydroxybenzylphosphonite: 98.5mol%;
[0057] Sodium bis(1-hydroxybenzyl)phosphonate: 1mol%;
[0058] Sodium 1-hydroxybenzylphosphonate: 0.5 mol%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com