Skin external preparation containing flurbiprofen
A technology of flurbiprofen and external preparations, applied in the field of poultice containing flurbiprofen, can solve problems such as poor hydrogel adhesion, and achieve the effect of solving poor adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
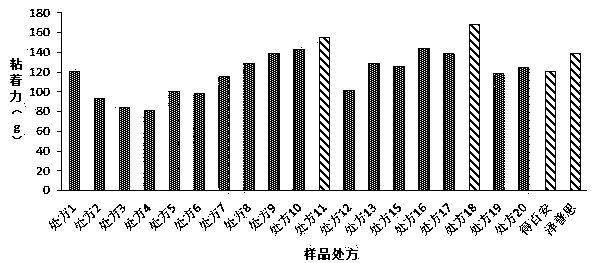
[0024] Example 1 Prescription Screening
[0025] Table 1. Sample formulation1
[0026]
[0027] Preparation:
[0028] Dissolve the prescribed amount of sodium carboxymethylcellulose in glycerin and partially purified water to obtain component A; dissolve the prescribed amount of gelatin in partially purified water, heat it after the expansion is complete, and stir until it becomes a transparent colloid to obtain the component A. Part B; dissolve the prescribed amount of polyacrylic acid-polyacrylic acid copolymer (NP700) and aluminum glycolate in glycerin, and stir evenly to obtain component C; mix the prescribed amount of flurbiprofen and crotamiton evenly , add L-menthol, isopropyl myristate (IPM), Tween 80, sorbitan sesquioleate, polyvinyl alcohol (PVA), polyacrylic acid and mix well to form component D; Disperse disodium acid and kaolin in purified water respectively, add components A, B, and C, fully knead, and disperse evenly, then add component D, use tartaric acid...
Embodiment 2
[0054] The research of embodiment 2 cross-linking agent
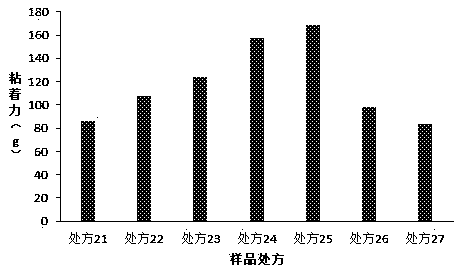
[0055] The present invention has carried out further research on the cross-linking agent in the prescription, and prescription is as table 3 below, and preparation method is as embodiment 1:
[0056] Table 3. Formulation Studies for Crosslinkers
[0057]
[0058] Determination of the above prescription and adhesion ( figure 2 ) results show that when aluminum hydroxide or aluminum glycolate is used as the crosslinking agent, the adhesion of babu plaster can achieve a satisfactory effect, especially when aluminum glycolate is used, the adhesion effect is the best. The consumption of joint agent is less than 0.2 part, and when greater than 4.0 part, all can not prepare the Babu ointment that meets the standard.
Embodiment 3
[0059] The research of embodiment 3 filler
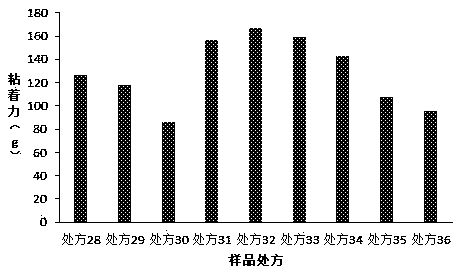
[0060] The present invention has carried out further research on the filling agent in the prescription, and the prescription is as in Table 3, and the preparation method is as in Example 1:
[0061] Table 4. Formulation Studies for Fillers
[0062]
[0063] Composed of the above prescription and the results of the adhesion test ( image 3 ) shows that both titanium dioxide and kaolin as fillers can effectively improve the adhesion, but their dosage must be strictly controlled. When the dosage exceeds 50 parts, the paste will be too hard to achieve effective coating. At 2.5 parts, the paste is soft and there will be residue when used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com