Preparation method of hyperbranched sulfonated polyether ether ketone
The technology of sulfonated polyether ether ketone and sulfonated fluoro ketone is applied in the field of preparation of hyperbranched sulfonated polyether ether ketone, and can solve the problems of decreased membrane resistance to alcohol, increased swelling rate of membrane materials, low stability and the like. Achieve the effect of protecting the interior of the material, increasing methanol resistance, and stabilizing three-dimensional space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A preparation method of hyperbranched sulfonated polyetheretherketone, comprising the following steps:
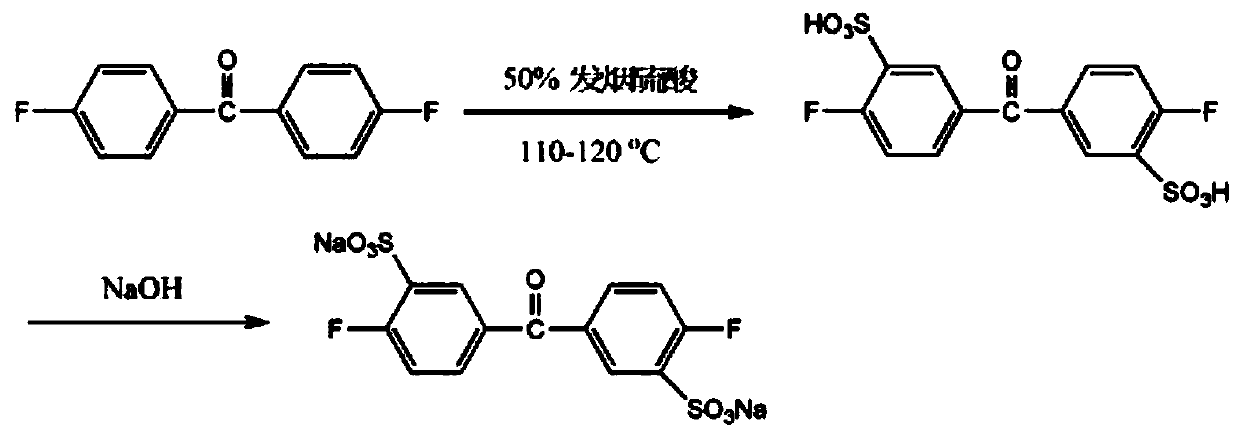
[0028] D1 prepares sulfonated difluorobenzophenone monomer
[0029] Sulfonate monomer difluorobenzophenone to obtain sulfonated fluoroketone monomer, put 50% oleum and difluorobenzophenone into a three-necked bottle, which is equipped with a mechanical stirring device, a thermometer and Spherical condenser, in the state of stirring, heated to 110 ° C, reacted for 6 hours, cooled to 40 ° C, slowly poured the reaction mixture into ice water, neutralized to neutral with sodium hydroxide solution, then added sodium chloride for salting out , to obtain a yellow-white solid crude product, recrystallized with ethanol and water to obtain 3,3-disulfonic acid-4,4'-difluorobenzophenone, referred to as sulfonated difluorobenzophenone monomer;
[0030] D2 Preparation of Hyperbranched Sulfonated Polyetheretherketone
[0031] 0.01mol of 2,4',6-trifluoro-benzophenone, 0.01mol of t...
Embodiment 2
[0033] A preparation method of hyperbranched sulfonated polyetheretherketone, comprising the following steps:
[0034] D1 prepares sulfonated difluorobenzophenone monomer
[0035] Sulfonate monomer difluorobenzophenone to obtain sulfonated fluoroketone monomer, put 50% oleum and difluorobenzophenone into a three-necked bottle, which is equipped with a mechanical stirring device, a thermometer and Spherical condenser, in the state of stirring, heated to 120 ° C, reacted for 8 hours, cooled to 50 ° C, slowly poured the reaction mixture into ice water, neutralized to neutral with sodium hydroxide solution, then added sodium chloride for salting out , to obtain a yellow-white solid crude product, recrystallized with ethanol and water to obtain 3,3-disulfonic acid-4,4'-difluorobenzophenone, referred to as sulfonated difluorobenzophenone monomer;
[0036] D2 Preparation of Hyperbranched Sulfonated Polyetheretherketone
[0037] 0.01mol of 2,4',6-trifluoro-benzophenone, 0.01mol of t...
Embodiment 3
[0039] A preparation method of hyperbranched sulfonated polyetheretherketone, comprising the following steps:
[0040] D1 prepares sulfonated difluorobenzophenone monomer
[0041]Sulfonate monomer difluorobenzophenone to obtain sulfonated fluoroketone monomer, put 50% oleum and difluorobenzophenone into a three-necked bottle, which is equipped with a mechanical stirring device, a thermometer and Spherical condenser, in the state of stirring, heated to 105 ° C, reacted for 7 hours, cooled to 45 ° C, slowly poured the reaction mixture into ice water, neutralized to neutral with sodium hydroxide solution, then added sodium chloride for salting out , to obtain a yellow-white solid crude product, recrystallized with ethanol and water to obtain 3,3-disulfonic acid-4,4'-difluorobenzophenone, referred to as sulfonated difluorobenzophenone monomer;
[0042] The principle of the reaction is an electrophilic substitution reaction of aromatics, and the structure of the reaction product i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com