Method for preparing 2-chloro-5-picoline
A technology of picoline and pyridone, which is applied in the field of chlorination to prepare 2-chloro-5-picoline, can solve the problems of side reactions, low selectivity and instability in the chlorination process, and achieve the effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
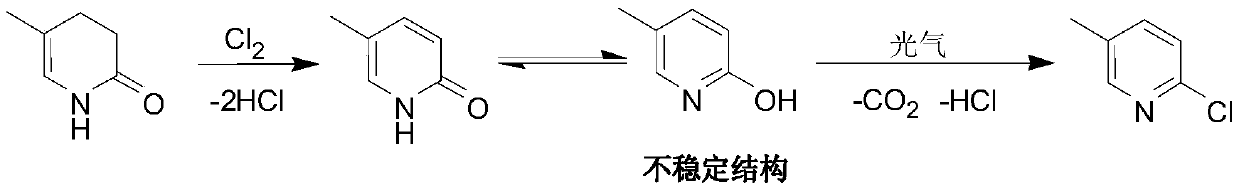
[0018] Raw material preparation: Weigh 1000g of pyridone with a content of 95%, put it into a four-necked bottle containing 1000g of chlorobenzene, stir to make it completely dissolve and set aside.
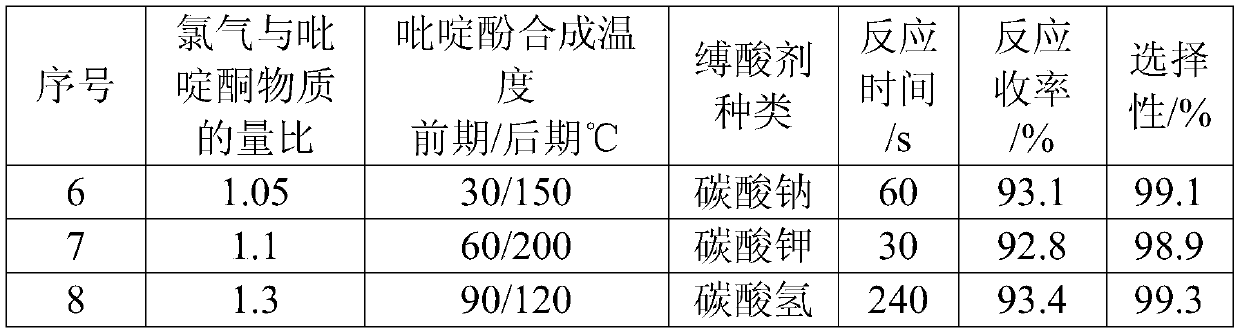
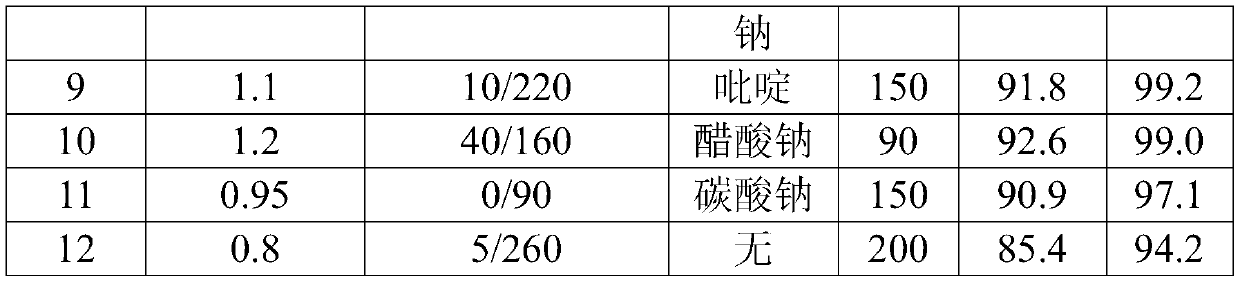
[0019] Synthesis of pyridinol: The reaction process adopts a continuous flow microchannel reactor, and feeds at the same time according to the ratio of 30% pyridone chlorobenzene liquid and chlorine gas substance of 1:1.05 as the raw material. The temperature of the first three plates is 50 ° C, and the temperature of the last three plates is 160 ° C. ℃, on the 4th plate pump into the quality of pyridone and other sodium carbonate aqueous solution (sodium carbonate content 10%). Controlling the residence time of reaction material by the flow rate of pump is 90s, and pyridinol chlorobenzene liquid discharges and collects continuously, adds DMF (accounting for 12% of pyridine weight) wherein to be used for next step reaction.
[0020] The synthesis of 2-chloro-5-picoline: the react...
Embodiment 2
[0022] (batch synthesis process)
[0023] Raw material preparation: Weigh 1000g of pyridone with a content of 95%, put it into a four-necked bottle containing 1000g of chlorobenzene, stir to make it completely dissolve and set aside.
[0024] Synthesis of pyridinol: put 225.2g of pyridone chlorobenzene solution (about 0.5mol of pyridone) into a 500mL four-necked bottle equipped with a thermometer and stirring, cool down to about 20°C and start to introduce chlorine gas, the flow rate of chlorine gas is 10L / h, and the reaction is controlled The temperature is 50°C, the molar ratio of chlorine gas to pyridone is 1.05:1, the reaction time is about 1h, then the temperature is raised to 160°C, and the temperature is kept for 2h to obtain pyridinol chlorobenzene liquid, to which DMF (accounting for 12% by weight of pyridine) is added ) for the next reaction.
[0025] Synthesis of 2-chloro-5-methylpyridine: Put 50g of chlorobenzene into a 500mL six-necked bottle equipped with a ther...
Embodiment 3
[0026] Embodiment 3 (adopting phosphorus oxychloride is chlorinating agent)
[0027] Raw material preparation: Weigh 1000g of pyridone with a content of 95%, put it into a four-necked bottle containing 1000g of chlorobenzene, stir to make it completely dissolve and set aside.
[0028] Synthesis of pyridinol: The reaction process adopts a continuous flow microchannel reactor, and feeds at the same time according to the ratio of 1:1.05 of the raw material pyridone chlorobenzene liquid and chlorine gas. The temperature of the first three plates is 50°C, and the temperature of the last three plates is 160°C. On the fourth plate, pump an aqueous solution of sodium carbonate (10% sodium carbonate content) equal to the quality of pyridone. Controlling the residence time of reaction material by the flow rate of pump is 90s, and pyridinol chlorobenzene liquid discharges and collects continuously, adds DMF (accounting for 12% of pyridine weight) wherein to be used for next step reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com